Predicting Mole-Fraction-Dependent Dissociation for Weak Acids
Graphical Abstract
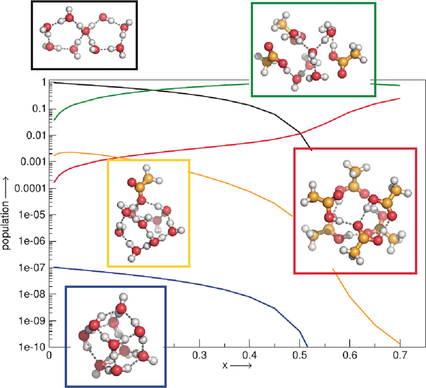
Binary quantum cluster equilibrium theory was applied to predict acid strengths over the complete concentration range through mole-fraction-depended populations of molecular and ionic clusters. It is shown for formic and acetic acid dissolved in water that the acid strength increases with increasing mole fraction in a complex way, reflecting the complex interplay between the dissociated hydronium ions.
Abstract
We demonstrate for formic and acetic acid dissolved in water as examples that the binary quantum cluster equilibrium (bQCE) approach can predict acid strengths over the whole range of acid concentrations. The acid strength increases in a complex rather than a simple way with increasing mole fraction of the acid from 0 to 0.7, reflecting the complex interplay between the dissociated ions or conjugate bases available as compared to the acid and water molecules. Furthermore, our calculated ion concentrations meet the experimental maximum of the conductivity with excellent agreement for acetic acid and satisfactorily for the formic acid/water mixture. As only a limited number of simple quantum-chemical calculations are required for the prediction, bQCE is clearly a valuable approach to access these quantities also in non-aqueous solutions. It is a highly valuable asset for predicting ionization processes in highly concentrated solutions, which are relevant for biological and chemical systems, as well as technological processes.