Design and Catalytic Asymmetric Construction of Axially Chiral 3,3′-Bisindole Skeletons
Chun Ma
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorFei Jiang
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Tao Sheng
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Yinchun Jiao
School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, 411201 P. R. China
Search for more papers by this authorDr. Guang-Jian Mei
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Shi
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorChun Ma
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorFei Jiang
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorFeng-Tao Sheng
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorDr. Yinchun Jiao
School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan, 411201 P. R. China
Search for more papers by this authorDr. Guang-Jian Mei
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Shi
School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P. R. China
Search for more papers by this authorGraphical Abstract
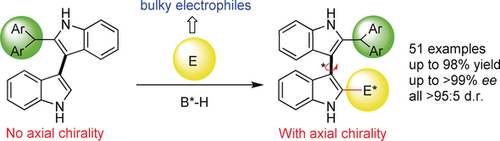
Bulk up: The first catalytic asymmetric construction of 3,3′-bisindole skeletons bearing axial and central chirality has been established by organocatalytic asymmetric addition reactions of 2-substituted 3,3′-bisindoles with electrophiles. This reaction also represents the first highly enantioselective construction of axially chiral 3,3′-bisindole skeletons, and utilizes the strategy of introducing a bulky group to the ortho-position of prochiral 3,3′-bisindoles.
Abstract
The first catalytic asymmetric construction of 3,3′-bisindole skeletons bearing both axial and central chirality has been established by organocatalytic asymmetric addition reactions of 2-substituted 3,3′-bisindoles with 3-indolylmethanols (up to 98 % yield, all >95:5 d.r., >99 % ee). This reaction also represents the first highly enantioselective construction of axially chiral 3,3′-bisindole skeletons, and utilizes the strategy of introducing a bulky group to the ortho-position of prochiral 3,3′-bisindoles. This reaction not only provides a good example for simultaneously controlling axial and central chirality in one operation, but also serves as a new strategy for catalytic enantioselective construction of axially chiral 3,3′-bisindole backbones from prochiral substrates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811177-sup-0001-misc_information.pdf25 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For some reviews, see:
- 1aM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193;
- 1bG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 1c Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weinheim, 2011.
- 2For an early review, see:
- 2aG. Bringmann, P. A. J. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384; Angew. Chem. 2005, 117, 5518; For some recent reviews:
- 2bJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 2cG. Bencivenni, Synlett 2015, 26, 1915;
- 2dR. M. Witzig, D. Lotter, V. C. Fäseke, C. Sparr, Chem. Eur. J. 2017, 23, 12960;
- 2eP. Renzi, Org. Biomol. Chem. 2017, 15, 4506;
- 2fY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534;
- 2gD. Bonne, J. Rodriguez, Chem. Commun. 2017, 53, 12385;
- 2hD. Bonne, J. Rodriguez, Eur. J. Org. Chem. 2018, 2417.
- 3For some recent examples on enantioselective synthesis of chiral biaryls, see:
- 3aP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131;
- 3bK. Mori, T. Itakura, T. Akiyama, Angew. Chem. Int. Ed. 2016, 55, 11642; Angew. Chem. 2016, 128, 11814;
- 3cH. Gao, Q.-L. Xu, C. Keene, M. Yousufuddin, D. H. Ess, L. Kürti, Angew. Chem. Int. Ed. 2016, 55, 566; Angew. Chem. 2016, 128, 576;
- 3dS. Staniland, R. W. Adams, J. J. W. McDouall, I. Maffucci, A. Contini, D. M. Grainger, N. J. Turner, J. Clayden, Angew. Chem. Int. Ed. 2016, 55, 10755; Angew. Chem. 2016, 128, 10913;
- 3eS. Shirakawa, X. Wu, S. Liu, K. Maruoka, Tetrahedron 2016, 72, 5163;
- 3fC. Xu, H. Zheng, B. Hu, X. Liu, L. Lin, X. Feng, Chem. Commun. 2017, 53, 9741.
- 4For some pioneering work on aryl–aryl couplings, see:
- 4aA. N. Cammidge, K. V. L. Cre'py, Chem. Commun. 2000, 1723;
- 4bJ. Yin, S. L. Buchwald, J. Am. Chem. Soc. 2000, 122, 12051;
- 4cQ.-X. Guo, Z.-J. Wu, Z.-B. Luo, Q.-Z. Liu, J.-L. Ye, S.-W. Luo, L.-F. Cun, L.-Z. Gong, J. Am. Chem. Soc. 2007, 129, 13927;
- 4dK. Yamaguchi, J. Yamaguchi, A. Studer, K. Itami, Chem. Sci. 2012, 3, 2165;
- 4eG.-Q. Li, H. Gao, C. Keene, M. Devonas, D. H. Ess, L. Kürti, J. Am. Chem. Soc. 2013, 135, 7414;
- 4fC. K. De, F. Pesciaioli, B. List, Angew. Chem. Int. Ed. 2013, 52, 9293; Angew. Chem. 2013, 125, 9463; For some recent work:
- 4gY.-H. Chen, D.-J. Cheng, J. Zhang, Y. Wang, X.-Y. Liu, B. Tan, J. Am. Chem. Soc. 2015, 137, 15062;
- 4hJ.-Z. Wang, J. Zhou, C. Xu, H. Sun, L. Kürti, Q.-L. Xu, J. Am. Chem. Soc. 2016, 138, 5202;
- 4iJ. Feng, B. Li, Y. He, Z. Gu, Angew. Chem. Int. Ed. 2016, 55, 2186; Angew. Chem. 2016, 128, 2226;
- 4jL. Ding, X. Sui, Z. Gu, ACS Catal. 2018, 8, 5630;
- 4kC. Pan, Z. Zhu, M. Zhang, Z. Gu, Angew. Chem. Int. Ed. 2017, 56, 4777; Angew. Chem. 2017, 129, 4855.
- 5For some representative work on functionalization of racemic or prochiral biaryls, see:
- 5aG. Bringmann, M. Breuning, R.-M. Pfeifer, W. A. Schenk, K. Kamikawa, M. Uemura, J. Organomet. Chem. 2002, 661, 31;
- 5bJ. L. Gustafson, D. Lim, S. J. Miller, Science 2010, 328, 1251;
- 5cK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964;
- 5dD.-J. Cheng, L. Yan, S.-K. Tian, M.-Y. Wu, L.-X. Wang, Z.-L. Fan, S.-C. Zheng, X.-Y. Liu, B. Tan, Angew. Chem. Int. Ed. 2014, 53, 3684; Angew. Chem. 2014, 126, 3758;
- 5eR. J. Armstrong, M. D. Smith, Angew. Chem. Int. Ed. 2014, 53, 12822; Angew. Chem. 2014, 126, 13036;
- 5fJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460;
- 5gG. Xu, W. Fu, G. Liu, C. H. Senanayake, W. Tang, J. Am. Chem. Soc. 2014, 136, 570;
- 5hC. Yu, H. Huang, X. Li, Y. Zhang, W. Wang, J. Am. Chem. Soc. 2016, 138, 6956;
- 5iK. Zhao, L. Duan, S. Xu, J. Jiang, Y. Fu, Z. Gu, Chem 2018, 4, 599.
- 6For some representative work on de novo construction of one or two aryl ring(s), see:
- 6aG. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. Int. Ed. 2007, 46, 3951; Angew. Chem. 2007, 119, 4025;
- 6bK. Tanaka, Chem. Asian J. 2009, 4, 508;
- 6cA. Link, C. Sparr, Angew. Chem. Int. Ed. 2014, 53, 5458; Angew. Chem. 2014, 126, 5562;
- 6dY.-B. Wang, S.-C. Zheng, Y.-M. Hu, B. Tan, Nat. Commun. 2017, 8, 15489;
- 6eY. Liu, X. Wu, S. Li, L. Xue, C. Shan, Z. Zhao, H. Yan, Angew. Chem. Int. Ed. 2018, 57, 6491; Angew. Chem. 2018, 130, 6601;
- 6fS. Jia, Z. Chen, N. Zhang, Y. Tan, Y. Liu, J. Deng, H. Yan, J. Am. Chem. Soc. 2018, 140, 7056.
- 7For some representative work on rearomatization of chiral non-aromatic bicyclic precursors, see:
- 7aF. Guo, L. C. Konkol, R. J. Thomson, J. Am. Chem. Soc. 2011, 133, 18;
- 7bO. Quinonero, M. Jean, N. Vanthuyne, C. Roussel, D. Bonne, T. Constantieux, C. Bressy, X. Bugaut, J. Rodriguez, Angew. Chem. Int. Ed. 2016, 55, 1401; Angew. Chem. 2016, 128, 1423.
- 8For some representative work, see:
- 8aV. Bhat, S. Wang, B. M. Stoltz, S. C. Virgil, J. Am. Chem. Soc. 2013, 135, 16829;
- 8bA. Ros, B. Estepa, P. Ramírez-López, E. Álvarez, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2013, 135, 15730;
- 8cD.-W. Gao, Q. Gu, S.-L. You, ACS Catal. 2014, 4, 2741;
- 8dJ.-W. Zhang, J.-H. Xu, D.-J. Cheng, C. Shi, X.-Y. Liu, B. Tan, Nat. Commun. 2016, 7, 10677;
- 8eS. Kinoshita, K. Kamikawa, Tetrahedron 2016, 72, 5202;
- 8fV. S. Raut, M. Jean, N. Vanthuyne, C. Roussel, T. Constantieux, C. Bressy, X. Bugaut, D. Bonne, J. Rodriguez, J. Am. Chem. Soc. 2017, 139, 2140.
- 9For some reviews, see:
- 9aG. R. Humphrey, J. T. Kuethe, Chem. Rev. 2006, 106, 2875;
- 9bM. Bandini, A. Eichholzer, Angew. Chem. Int. Ed. 2009, 48, 9608; Angew. Chem. 2009, 121, 9786;
- 9cA. J. Kochanowska-Karamyan, M. T. Hamann, Chem. Rev. 2010, 110, 4489.
- 10
- 10aH.-H. Zhang, C.-S. Wang, C. Li, G.-J. Mei, Y. Li, F. Shi, Angew. Chem. Int. Ed. 2017, 56, 116; Angew. Chem. 2017, 129, 122;
- 10bL.-W. Qi, J.-H. Mao, J. Zhang, B. Tan, Nat. Chem. 2018, 10, 58;
- 10cC. He, M. Hou, Z. Zhu, Z. Gu, ACS Catal. 2017, 7, 5316.
- 11For some reviews, see:
- 11aA. Steven, L. E. Overman, Angew. Chem. Int. Ed. 2007, 46, 5488; Angew. Chem. 2007, 119, 5584;
- 11bP. Ruiz-Sanchis, S. A. Savina, F. Albericio, M. Alvarez, Chem. Eur. J. 2011, 17, 1388. For some examples:
- 11cJ. Song, C. Guo, A. Adele, H. Yin, L. Z. Gong, Chem. Eur. J. 2013, 19, 3319;
- 11dC. R. Jamison, J. J. Badillo, J. M. Lipshultz, R. J. Comito, D. W. C. MacMillan, Nat. Chem. 2017, 9, 1165.
- 12For some examples, see:
- 12aY. Li, W.-H. Wang, S.-D. Yang, B.-J. Li, C. Feng, Z.-J. Shi, Chem. Commun. 2010, 46, 4553;
- 12bC. Ramesh, V. Kavala, C.-W. Kuo, B. R. Raju, C.-F. Yao, Eur. J. Org. Chem. 2010, 3796;
- 12cJ. E. Perea-Buceta, T. Wirtanen, O. V. Laukkanen, M. K. Makela, M. Nieger, M. Melchionna, N. Huittinen, J. A. Lopez-Sanchez, J. Helaja, Angew. Chem. Int. Ed. 2013, 52, 11835; Angew. Chem. 2013, 125, 12051;
- 12dA. Arcadi, M. Chiarini, G. D'Anniballe, F. Marinelli, E. Pietropaolo, Org. Lett. 2014, 16, 1736;
- 12eN. H. Ansari, C. A. Dacko, N. G. Akhmedov, B. C. Soderberg, J. Org. Chem. 2016, 81, 9337;
- 12fY.-Y. He, X.-X. Sun, G.-H. Li, G.-J. Mei, F. Shi, J. Org. Chem. 2017, 82, 2462;
- 12gT.-R. Li, M.-M. Zhang, B.-C. Wang, L.-Q. Lu, W.-J. Xiao, Org. Lett. 2018, 20, 3237.
- 13For a report on resolution of axially chiral 3,3′-bisindole derivatives, see: U. Berens, J. M. Brown, J. Long, R. Selke, Tetrahedron: Asymmetry 1996, 7, 285; There are only two examples on catalytic asymmetric construction of axially chiral 3,3′-bisindole scaffold but with low or moderate enantioselectivity: References [10a] and [12g].
- 14
- 14aY.-C. Zhang, J.-J. Zhao, F. Jiang, S.-B. Sun, F. Shi, Angew. Chem. Int. Ed. 2014, 53, 13912; Angew. Chem. 2014, 126, 14132;
- 14bJ.-J. Zhao, S.-B. Sun, S.-H. He, Q. Wu, F. Shi, Angew. Chem. Int. Ed. 2015, 54, 5460; Angew. Chem. 2015, 127, 5550;
- 14cC. Ma, J.-Y. Zhou, Y.-Z. Zhang, G.-J. Mei, F. Shi, Angew. Chem. Int. Ed. 2018, 57, 5398; Angew. Chem. 2018, 130, 5496.
- 15For some reviews, see:
- 15aT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 15bM. Terada, Chem. Commun. 2008, 35, 4097;
- 15cM. Terada, Synthesis 2010, 1929;
- 15dA. Zamfir, S. Schenker, M. Freund, S. B. Tsogoeva, Org. Biomol. Chem. 2010, 8, 5262;
- 15eJ. Yu, F. Shi, L.-Z. Gong, Acc. Chem. Res. 2011, 44, 1156;
- 15fD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 15gH. Wu, Y.-P. He, F. Shi, Synthesis 2015, 47, 1990;
- 15hF. E. Held, D. Grau, S. B. Tsogoeva, Molecules 2015, 20, 16103.
- 16For some reviews, see:
- 16aA. Palmieri, M. Petrini, R. R. Shaikh, Org. Biomol. Chem. 2010, 8, 1259;
- 16bL. Chen, X.-P. Yin, C.-H. Wang, J. Zhou, Org. Biomol. Chem. 2014, 12, 6033;
- 16cY. Chen, L. Wang, J. Xiao, Asian J. Org. Chem. 2014, 3, 1036;
- 16dG.-J. Mei, F. Shi, J. Org. Chem. 2017, 82, 7695.
- 17For some early examples on catalytic asymmetric reactions of 3-indoylmethanols, see:
- 17aQ.-X. Guo, Y.-G. Peng, J.-W. Zhang, L. Song, Z. Feng, L.-Z. Gong, Org. Lett. 2009, 11, 4620;
- 17bF.-L. Sun, M. Zeng, Q. Gu, S.-L. You, Chem. Eur. J. 2009, 15, 8709;
- 17cP. G. Cozzi, F. Benfatti, L. Zoli, Angew. Chem. Int. Ed. 2009, 48, 1313; Angew. Chem. 2009, 121, 1339.
- 18For some examples, see:
- 18aT. Shibata, M. Otomo, Y. Tahara, K. Endo, Org. Biomol. Chem. 2008, 6, 4296;
- 18bN. Di Iorio, P. Righi, A. Mazzanti, M. Mancinelli, A. Ciogli, G. Bencivenni, J. Am. Chem. Soc. 2014, 136, 10250;
- 18cZ. Zuo, J. Liu, J. Nan, L. Fan, W. Sun, Y. Wang, X. Luan, Angew. Chem. Int. Ed. 2015, 54, 15385; Angew. Chem. 2015, 127, 15605;
- 18dH.-C. Liu, H.-Y. Tao, H. Cong, C.-J. Wang, J. Org. Chem. 2016, 81, 3752;
- 18eC. Min, Y. Lin, D. Seidel, Angew. Chem. Int. Ed. 2017, 56, 15353; Angew. Chem. 2017, 129, 15555;
- 18fY. Liu, Y.-L. S. Tse, F. Y. Kwong, Y.-Y. Yeung, ACS Catal. 2017, 7, 4435;
- 18gV. Hornillos, J. A. Carmona, A. Ros, J. Iglesias-Sigüenza, J. López-Serrano, R. Fernández, J. M. Lassaletta, Angew. Chem. Int. Ed. 2018, 57, 3777; Angew. Chem. 2018, 130, 3839;
- 18hJ. Liu, X. Yang, Z. Zuo, J. Nan, Y. Wang, X. Luan, Org. Lett. 2018, 20, 244;
- 18iJ. A. Carmona, V. Hornillos, P. Ramírez-López, A. Ros, J. Iglesias-Sigüenza, E. Gómez-Bengoa, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2018, 140, 11067;
- 18jY.-S. Jang, Ł. Woźniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901; Angew. Chem. 2018, 130, 13083.
- 19CCDC 1866978 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. See the Supporting Information.