Bimetallic Rhodium(II)/Indium(III) Relay Catalysis for Tandem Insertion/Asymmetric Claisen Rearrangement
Yushuang Chen
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Shunxi Dong
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXi Xu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorYushuang Chen
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Shunxi Dong
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXi Xu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorGraphical Abstract
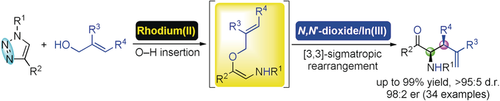
A highly efficient asymmetric tandem reaction of N-sulfonyl-1,2,3-triazoles with two types of allyl alcohol esters was achieved by using a bimetallic rhodium(II)/chiral N,N′-dioxide–indium(III) complex catalyst. A series of β/γ-amino acid derivatives bearing different substituents were obtained in a good to excellent diastereo- and enantioselective manner.
Abstract
The first example of catalytic insertion/asymmetric Claisen rearrangement tandem reaction of N-sulfonyl-1,2,3-triazoles with allyl alcohol esters was achieved by bimetallic relay catalytic systems involving achiral rhodium salt and chiral N,N′-dioxide–indium(III) complex. This manifold could overcome the limitation of single RhII catalysis, providing a straight and facile route to various enantioenriched β/γ-amino acid derivatives in high yields (up to 99 %) with excellent diastereo- and enantioselectivities (up to >95:5 dr, 98:2 er). Moreover, possible transition state models were proposed to elucidate the origin of chiral induction based on the control experiments and X-ray crystal structure of the catalyst.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810410-sup-0001-misc_information.pdf17.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on the asymmetric reactions of metal carbenes derived from diazo compounds, see:
- 1aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861;
- 1bM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704;
- 1cS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365;
- 1dH. M. L. Davies, Y. J. Lian, Acc. Chem. Res. 2012, 45, 923;
- 1eX. Guo, W. H. Hu, Acc. Chem. Res. 2013, 46, 2427;
- 1fS. Chanthamath, S. Iwasa, Acc. Chem. Res. 2016, 49, 2080;
- 1gY. Xia, D. Qiu, J. B. Wang, Chem. Rev. 2017, 117, 13810.
- 2For selected examples on the asymmetric reactions of metal carbenes derived from diazo compounds, see:
- 2aG. A. Moniz, J. L. Wood, J. Am. Chem. Soc. 2001, 123, 5095;
- 2bZ. J. Li, H. M. L. Davies, J. Am. Chem. Soc. 2010, 132, 396;
- 2cS.-F. Zhu, Y. Cai, H.-X. Mao, J.-H. Xie, Q.-L. Zhou, Nat. Chem. 2010, 2, 546;
- 2dZ. J. Li, V. Boyarskikh, J. H. Hansen, J. Autschbach, D. G. Musaev, H. M. L. Davies, J. Am. Chem. Soc. 2012, 134, 15497;
- 2eX. C. Xu, P. Y. Zavalij, M. P. Doyle, J. Am. Chem. Soc. 2013, 135, 12439;
- 2fZ. K. Zhang, Z. Sheng, W. Z. Yu, G. J. Wu, R. Zhang, W. D. Chu, Y. Zhang, J. B. Wang, Nat. Chem. 2017, 9, 970;
- 2gX. B. Lin, Y. Tang, W. Yang, F. Tan, L. L. Lin, X. H. Liu, X. M. Feng, J. Am. Chem. Soc. 2018, 140, 3299.
- 3
- 3aS. Chuprakov, F. W. Hwang, V. Gevorgyan, Angew. Chem. Int. Ed. 2007, 46, 4757; Angew. Chem. 2007, 119, 4841;
- 3bB. Chattopadhyay, V. Gevorgyan, Angew. Chem. Int. Ed. 2012, 51, 862; Angew. Chem. 2012, 124, 886;
- 3cY. Shi, A. V. Gulevich, V. Gevorgyan, Angew. Chem. Int. Ed. 2014, 53, 14191; Angew. Chem. 2014, 126, 14415;
- 3dJ. H. Kim, T. Gensch, D. B. Zhao, L. Stegemann, C. A. Strassert, F. Glorius, Angew. Chem. Int. Ed. 2015, 54, 10975; Angew. Chem. 2015, 127, 11126;
- 3eA. Joshi, D. C. Mohan, S. Adimurthy, Org. Lett. 2016, 18, 464.
- 4
- 4aH. M. L. Davies, J. S. Alford, Chem. Soc. Rev. 2014, 43, 5151;
- 4bP. Anbarasan, D. Yadagiri, S. Rajasekar, Synthesis 2014, 46, 3004;
- 4cY. Jiang, R. Sun, X. Y. Tang, M. Shi, Chem. Eur. J. 2016, 22, 17910;
- 4dT. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan, V. V. Fokin, J. Am. Chem. Soc. 2008, 130, 14972;
- 4eT. Miura, T. Biyajima, T. Fujii, M. Murakami, J. Am. Chem. Soc. 2012, 134, 194;
- 4fS. Y. Liu, W. F. Yao, Y. Liu, Q. H. Wei, J. H. Chen, X. Wu, F. Xia, W. H. Hu, Sci. Adv. 2017, 3, e 1602467.
- 5T. Nakamuro, K. Hagiwara, T. Miura, M. Murakami, Angew. Chem. Int. Ed. 2018, 57, 5497; Angew. Chem. 2018, 130, 5595.
- 6
- 6aT. Miura, T. Tanaka, T. Biyajima, A. Yada, M. Murakami, Angew. Chem. Int. Ed. 2013, 52, 3883; Angew. Chem. 2013, 125, 3975;
- 6bT. Miura, T. Tanaka, K. Matsumoto, M. Murakami, Chem. Eur. J. 2014, 20, 16078;
- 6cD. J. Jung, H. J. Jeon, J. H. Lee, S.-g. Lee, Org. Lett. 2015, 17, 3498;
- 6dH. J. Jeon, M. S. Kwak, D. J. Jung, J. Bouffard, S.-g. Lee, Org. Biomol. Chem. 2016, 14, 11238;
- 6eT. Miura, T. Tanaka, Q. Zhao, S. G. Stewart, M. Murakami, Helv. Chim. Acta. 2017, 100, e 1600320;
- 6fD. Yadagiri, P. Anbarasan, Chem. Eur. J. 2013, 19, 15115;
- 6gT. Miura, T. Tanaka, A. Yada, M. Murakami, Chem. Lett. 2013, 42, 1308.
- 7For selected examples on the asymmetric reactions of 1,2,3-triazoles, see:
- 7aS. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher, V. V. Fokin, J. Am. Chem. Soc. 2009, 131, 18034;
- 7bN. Grimster, L. Zhang, V. V. Fokin, J. Am. Chem. Soc. 2010, 132, 2510;
- 7cT. Miura, T. Nakamuro, H. Nikishima, M. Murakami, Chem. Lett. 2016, 45, 1003;
- 7dS. Chuprakov, J. A. Malik, M. Zibinsky, V. V. Fokin, J. Am. Chem. Soc. 2011, 133, 10352;
- 7eR. W. Kubiak II, J. D. Mighion, S. M. Wilkerson-Hill, J. S. Alford, T. Yoshidomi, H. M. L. Davies, Org. Lett. 2016, 18, 3118;
- 7fJ. E. Spangler, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 6802;
- 7gM. Zibinsky, V. V. Fokin, Angew. Chem. Int. Ed. 2013, 52, 1507; Angew. Chem. 2013, 125, 1547;
- 7hR. W. Kubiak II, H. M. L. Davies, Org. Lett. 2018, 20, 3771.
- 8
- 8aM. Seki, T. Shimizu, K. Matsumoto, J. Org. Chem. 2000, 65, 1298;
- 8bA. E. Taggi, A. M. Hafez, T. Lectka, Acc. Chem. Res. 2003, 36, 10;
- 8cA. R. Katritzky, H. Tao, R. Jiang, K. Suzuki, K. Kirichenko, J. Org. Chem. 2007, 72, 407;
- 8dK. K. Zhang, X. P. Liang, M. He, J. Wu, Y. P. Zhang, W. Xue, L. H. Jin, S. Yang, D. Y. Hu, Molecules 2013, 18, 6142.
- 9
- 9aX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574;
- 9bX. H. Liu, L. L. Lin, X. M. Feng, Org. Chem. Front. 2014, 1, 298;
- 9cX. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2017, 50, 2621;
- 9dX. H. Liu, S. X. Dong, L. L. Lin, X. M. Feng, Chin. J. Chem. 2018, 36, 791;
- 9eX. M. Feng, Z. Wang, X. H. Liu, Top. Organomet. Chem. 2018, 62, 147.
- 10
- 10aJ. Li, L. L. Lin, B. W. Hu, X. J. Lian, G. Wang, X. H. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2016, 55, 6075; Angew. Chem. 2016, 128, 6179;
- 10bJ. Li, L. L. Lin, B. W. Hu, P. F. Zhou, T. Y. Huang, X. H. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2017, 56, 885; Angew. Chem. 2017, 129, 903;
- 10cT. Y. Huang, X. H. Liu, J. W. Lang, J. Xu, L. L. Lin, X. M. Feng, ACS Catal. 2017, 7, 5654;
- 10dD. Zhang, L. L. Lin, J. Yang, X. H. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2018, 57, 12323; Angew. Chem. 2018, 130, 12503;
- 10eB. W. Hu, J. Li, W. D. Cao, Q. C. Lin, J. Yang, L. L. Lin, X. H. Liu, X. M. Feng, Adv. Synth. Catal. 2018, 360, 2831;
- 10fJ. Yang, C. Q. Ke, D. Zhang, X. H. Liu, X. M. Feng, Org. Lett. 2018, 20, 4536.
- 11
- 11aA. M. Martín Castro, Chem. Rev. 2004, 104, 2939;
- 11bE. A. Ilardi, C. E. Stivala, A. Zakarian, Chem. Soc. Rev. 2009, 38, 3133;
- 11cY. B. Liu, X. H. Liu, H. P. Hu, J. Guo, Y. Xia, L. L. Lin, X. M. Feng, Angew. Chem. Int. Ed. 2016, 55, 4054; Angew. Chem. 2016, 128, 4122;
- 11dH. F. Zheng, Y. Wang, C. R. Xu, X. Xu, L. L. Lin, X. H. Liu, X. M. Feng, Nat. Commun. 2018, 9, 1968.
- 12Several rhodium sources were tested, and Rh2(esp)2 was shown to be the most efficient one (Table S1 in the Supporting Information).
- 13With In(OTf)3 as the central metal, selected chiral N,N′-dioxide ligands were also investigated, no better results were obtained.
- 14CCDC 1846270, 1846269, and 1866880 (3 ab, 3 ag, and [L3-TQ-(S)-EPh/In(OTf)3]) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 15
- 15aK. M. Osten, P. Mehrkhodavandi, Acc. Chem. Res. 2017, 50, 2861;
- 15bI. Yu, A. Acosta-Ramírez, P. Mehrkhodavandi, J. Am. Chem. Soc. 2012, 134, 12758;
- 15cA. Acosta-Ramírez, A. F. Douglas, I. Yu, B. O. Patrick, P. L. Diaconescu, P. Mehrkhodavandi, Inorg. Chem. 2010, 49, 5444;
- 15dA. F. Douglas, B. O. Patrick, P. Mehrkhodavandi, Angew. Chem. Int. Ed. 2008, 47, 2290; Angew. Chem. 2008, 120, 2322.