Applications of Selenonium Cations as Lewis Acids in Organocatalytic Reactions
Xinxin He
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorXinyan Wang
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorProf. Dr. Ying-Lung (Steve) Tse
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorCorresponding Author
Dr. Zhihai Ke
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorXinxin He
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorXinyan Wang
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorProf. Dr. Ying-Lung (Steve) Tse
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorCorresponding Author
Dr. Zhihai Ke
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong, China
Search for more papers by this authorDedicated to Professor Elias J. Corey on the occasion of his 90th birthday
Graphical Abstract
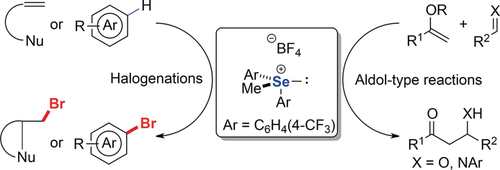
Se salt: The use of trisubstituted selenonium salts as organic Lewis acids in electrophilic halogenation and aldol-type reactions has been developed. The substrate scope is broad. The reaction conditions are mild and compatible with various functionalities. This study opens a new avenue for the development of non-metallic Lewis acid catalysis.
Abstract
The use of trisubstituted selenonium salts as organic Lewis acids in electrophilic halogenation and aldol-type reactions has been developed. The substrate scope is broad. The reaction conditions are mild and compatible with various functionalities. This study opens a new avenue for the development of nonmetallic Lewis acid catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201806965-sup-0001-misc_information.pdf4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. M. McGarrigle, E. L. Myers, O. Illa, M. A. Shaw, S. L. Riches, V. K. Aggarwal, Chem. Rev. 2007, 107, 5841–5883;
- 1bS. E. Denmark, G. L. Beutner, Angew. Chem. Int. Ed. 2008, 47, 1560–1638; Angew. Chem. 2008, 120, 1584–1663.
- 2 Lewis Base Catalysis in Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2016.
- 3
- 3aS. Benz, J. Mareda, C. Besnard, N. Sakai, S. Matile, Chem. Sci. 2017, 8, 8164–8169;
- 3bP. Wonner, L. Vogel, M. Düser, L. Gomers, F. Kniep, B. Mallick, D. B. Werz, S. M. Huber, Angew. Chem. Int. Ed. 2017, 56, 12009–12012; Angew. Chem. 2017, 129, 12172–12176;
- 3cS. Benz, J. Lopez-Andarias, J. Mareda, N. Sakai, S. Matile, Angew. Chem. Int. Ed. 2017, 56, 812–815; Angew. Chem. 2017, 129, 830–833;
- 3dS. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai, S. Matile, J. Am. Chem. Soc. 2016, 138, 9093–9096.
- 4H. Takada, P. Metzner, C. Philouze, Chem. Commun. 2001, 2350–2351.
- 5
- 5aZ. Ke, C. K. Tan, F. Chen, Y.-Y. Yeung, J. Am. Chem. Soc. 2014, 136, 5627–5630;
- 5bF. Chen, C. K. Tan, Y.-Y. Yeung, J. Am. Chem. Soc. 2013, 135, 1232–1235.
- 6
- 6aC. B. Caputo, L. J. Hounjet, R. Dobrovetsky, D. W. Stephan, Science 2013, 341, 1374–1377;
- 6bT. Werner, Adv. Synth. Catal. 2009, 351, 1469–1481.
- 7
- 7aY. Kim, M. Kim, F. P. Gabbaï, Org. Lett. 2010, 12, 600–602;
- 7bH. Zhao, F. Gabbaï, Nat. Chem. 2010, 2, 984–990.
- 8
- 8aE. J. Corey, M. Chaykovsky, J. Am. Chem. Soc. 1965, 87, 1353;
- 8bY. G. Gololobov, V. P. Lysenko, I. E. Boldeskul, Tetrahedron 1987, 43, 2609;
- 8c Encyclopedia of Reagents for Organic Synthesis (Eds.: ), Wiley, New York, 2006;
- 8dE. J. Lenardão, S. R. Mendes, P. C. Ferreira, G. Perin, C. C. Silveirab, R. G. Jacoba, Tetrahedron Lett. 2006, 47, 7439–7442;
- 8eJ. L. Dektar, N. P. Hacker, J. Am. Chem. Soc. 1990, 112, 6004–6015;
- 8fV. P. Singh, H. B. Singh, R. J. Butcher, Eur. J. Inorg. Chem. 2010, 637–647.
- 9
- 9aK. P. Bhabak, G. Mugesh, Acc. Chem. Res. 2010, 43, 1408–1419;
- 9bJ. C. Taylor, G. D. Markham, J. Biol. Chem. 1999, 274, 32909–32914;
- 9cF. T. Burling, B. M. Goldstein, J. Am. Chem. Soc. 1992, 114, 2313–2320;
- 9dY. Nagao, T. Hirata, S. Goto, S. Sano, A. Kakehi, K. Iizuka, M. Shiro, J. Am. Chem. Soc. 1998, 120, 3104–3110;
- 9eJ. C. Graham, C. H. Feng, M. Orticochea, G. Ahmed, J. Org. Chem. 1990, 55, 4102–4109;
- 9fC. Jo, S. Lee, S. J. Cho, R. Ryoo, Angew. Chem. Int. Ed. 2015, 54, 12805–12808; Angew. Chem. 2015, 127, 12996–12999;
- 9gK. T. Mahmudov, M. N. Kopylovich, M. F. C. Guedes da Silva, A. J. L. Pombeiro, Dalton Trans. 2017, 46, 10121–10138;
- 9hW. Z. Wang, B. M. Ji, Y. Zhang, J. Phys. Chem. A 2009, 113, 8132–8135.
- 10T. K. Green, P. Whitley, K. Wu, W. G. Lloyd, L. Z. Gan, Energy Fuels 1994, 8, 244–248.
- 11The details appear in the Supporting Information.
- 12CCDC 1840887 and 1841660 (6 a and 6 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 13
- 13a Chemistry of Hypervalent Compounds (Ed.: ), Wiley-VCH, Weinheim, 1999, pp. 211–278;
- 13bK. A. Leonard, F. Zhou, M. R. Detty, Organometallics 1996, 15, 4285–4292.
- 14The effect of the tetrafluoroborate anion on the chemical shift was found to be negligible. For details, see Figure S2 in the supporting Information.
- 15
- 15aH. Komatsu, M. Iwaoka, S. Tomoda, Chem. Commun. 1999, 205–206;
- 15bS. S. Zade, S. Panda, H. B. Singh, R. B. Sunoj, R. J. Butcher, J. Org. Chem. 2005, 70, 3693–3704;
- 15cM. Iwaoka, S. Tomoda, Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 755–766.
- 16Density-functional theory (DFT) calculations on the putative 6 b-DMA adduct were carried out using M06-2X hybrid-exchange correlation functional with Grimme D3 dispersion correction and def2-TZVP basis set by Gaussian 09. For details, Figures S13 and S14 in the Supporting Information.
- 17
- 17aS. W. Benson, Chem. Rev. 1978, 78, 23–35;
- 17bR. J. Arhart, J. C. Martin, J. Am. Chem. Soc. 1972, 94, 5003–5010;
- 17cS. J. Balkrishna, C. D. Prasad, P. Panini, M. R. Detty, D. Chopra, S. Kumar, J. Org. Chem. 2012, 77, 9541–9552.
- 18L. Racicot, T. Kasahara, M. A. Ciufolini, Org. Lett. 2014, 16, 6382–6385.
- 19Although the catalytic performance of 6 c was better than that of 6 b, 6 c was relatively more hygroscopic and difficult to handle. Thus, 6 b was used in other experimentations.
- 20
- 20aY.-C. Wong, Z. Ke, Y.-Y. Yeung, Org. Lett. 2015, 17, 4944–4947;
- 20bC. Rösner, U. Hennecke, Org. Lett. 2015, 17, 3226–3229;
- 20cZ. Ke, Y.-C. Wong, J. Y. See, Y.-Y. Yeung, Adv. Synth. Catal. 2016, 358, 1719–1724.
- 21I. Saikia, A. J. Borah, P. Phukan, Chem. Rev. 2016, 116, 6837–7042.
- 22
- 22a Handbook of Cyclization Reactions, Vol. 4 (Eds.: ), Wiley-VCH, Weinheim, 2010, pp. 951–990;
- 22bS. Ranganathan, K. M. Muraleedharan, N. K. Vaish, N. Jayaraman, Tetrahedron 2004, 60, 5273–5308;
- 22cS. R. Chemler, M. T. Bovino, ACS Catal. 2013, 3, 1076–1091.
- 23
- 23aK. Mikami, M. Shimuzu, Chem. Rev. 1992, 92, 1021–1050;
- 23bD. J. Berrisford, C. Bolm, Angew. Chem. Int. Ed. Engl. 1995, 34, 1717–1719; Angew. Chem. 1995, 107, 1862–1864;
- 23cJ. S. Johnson, D. A. Evans, Acc. Chem. Res. 2000, 33, 325–335;
- 23dM. L. Clarke, M. B. France, Tetrahedron 2008, 64, 9003–9031.
- 24
- 24aJ. Matsuo, M. Murakami, Angew. Chem. Int. Ed. 2013, 52, 9109–9118; Angew. Chem. 2013, 125, 9280–9289;
- 24bS. B. J. Kan, K. K.-H. Ng, I. Paterson, Angew. Chem. Int. Ed. 2013, 52, 9097–9108; Angew. Chem. 2013, 125, 9267–9279;
- 24cT. Kitanosono, S. Kobayashi, Adv. Synth. Catal. 2013, 355, 3095–3118.
- 25
- 25aB. B. Thompson, J. Pharm. Sci. 1968, 57, 715–733;
- 25bW. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580–591;
- 25cJ. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg, F. P. J. T. Rutjes, Chem. Soc. Rev. 2008, 37, 29–41;
- 25dA. Moyano, R. Rios, Chem. Rev. 2011, 111, 4703–4832.