Catalytic Asymmetric Mannich Reaction with N-Carbamoyl Imine Surrogates of Formaldehyde and Glyoxylate
Yang'en You
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorDr. Long Zhang
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorLinfeng Cui
College of Chemistry, Beijing Normal University, Beijing, 100875 China
Search for more papers by this authorProf. Xueling Mi
College of Chemistry, Beijing Normal University, Beijing, 100875 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Sanzhong Luo
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorYang'en You
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorDr. Long Zhang
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorLinfeng Cui
College of Chemistry, Beijing Normal University, Beijing, 100875 China
Search for more papers by this authorProf. Xueling Mi
College of Chemistry, Beijing Normal University, Beijing, 100875 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Sanzhong Luo
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100490 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
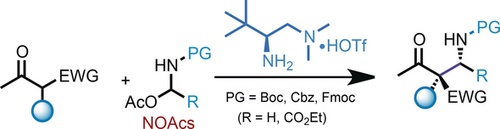
On the bench: N,O-acetals (NOAcs) were developed as bench-stable surrogates for N-carbamoyl (Boc, Cbz, Fmoc) formaldehyde and glyoxylate imines in asymmetric Mannich reactions. This reaction offers a straightforward approach for the asymmetric synthesis of α- or β-amino carbonyls bearing chiral quaternary centers in a practical and highly stereocontrolled manner. EWG=electron-withdrawing group, PG=protecting group.
Abstract
N,O-acetals (NOAcs) were developed as bench stable surrogates for N-carbamoyl, (Boc, Cbz and Fmoc) formaldehyde and glyoxylate imines in asymmetric Mannich reactions. The NOAcs can be directly utilized in the chiral primary amine catalyzed Mannich reactions of both acyclic and cyclic β-ketocarbonyls with high yields and excellent stereoselectivity. The current reaction offers a straightforward approach in the asymmetric synthesis of α- or β-amino carbonyls bearing chiral quaternary centers in a practical and highly stereocontrolled manner.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201707005-sup-0001-misc_information.pdf8.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Mannich, W. Krösche, Arch. Pharm. 1912, 250, 647.
- 2
- 2aH. Ishitani, M. Ueno, S. Kobayashi, J. Am. Chem. Soc. 1997, 119, 7153;
- 2bB. List, J. Am. Chem. Soc. 2000, 122, 9336;
- 2cW. Notz, K. Sakthivel, T. Bui, G. Zhong, C. F. Barbas III, Tetrahedron Lett. 2001, 42, 199;
- 2dFor an early review, see: S. Kobayashi, H. Ishitani, Chem. Rev. 1999, 99, 1069;
- 2eS. Kobayashi, Y. Mori, J. S. Fossey, M. M. Salter, Chem. Rev. 2011, 111, 2626.
- 3Recent reviews on Mannich reaction:
- 3aW. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580;
- 3bA. Córdova, Acc. Chem. Res. 2004, 37, 102;
- 3cY. Liu, Q. Wu, D. Yin, D. Li, Chin. J. Org. Chem. 2016, 36, 927;
- 3dM. S. Roselló, C. Pozo, S. Fustero, Synthesis 2016, 48, 2553;
- 3eR. G. Arrayás, J. C. Carretero, Chem. Soc. Rev. 2009, 38, 1940.
- 4
- 4aY. Nakamura, R. Matsubara, H. Kiyohara, S. Kobayashi, Org. Lett. 2003, 5, 2481;
- 4bM. Wasa, R. Y. Liu, S. P. Roche, E. N. Jacobsen, J. Am. Chem. Soc. 2014, 136, 12872.
- 5
- 5aJ. Song, H. W. Shih, L. Deng, Org. Lett. 2007, 9, 603;
- 5bF. Fini, V. Sgarzani, D. Pettersen, R. P. Herrera, L. Bernardi, A. Ricci, Angew. Chem. Int. Ed. 2005, 44, 7975; Angew. Chem. 2005, 117, 8189;
- 5cC. Palomo, M. Oiarbide, A. Laso, R. López, J. Am. Chem. Soc. 2005, 127, 17622;
- 5dC. Gianelli, L. Sambri, A. Carlone, G. Bartoli, P. Melchiorre, Angew. Chem. Int. Ed. 2008, 47, 8700; Angew. Chem. 2008, 120, 8828.
- 6
- 6aA. E. Hartman, C. L. Brophy, J. A. Cupp, D. K. Hodge, T. J. Peelen, J. Org. Chem. 2009, 74, 3952;
- 6bS. P. Roche, S. S. Samanta, M. M. J. Gosselin, Chem. Commun. 2014, 50, 2632;
- 6cA. B. Weinstein, D. P. Schuman, Z. X. Tan, S. S. Stahl, Angew. Chem. Int. Ed. 2013, 52, 11867; Angew. Chem. 2013, 125, 12083;
- 6dD. Ferraris, B. Young, T. Dudding, W. J. Drury III, T. Lectka, Tetrahedron 1999, 55, 8869;
- 6eN. N. Nasief, D. Hangauer, J. Med. Chem. 2014, 57, 2315.
- 7Y.-Y. Huang, C. Cai, X. Yang, Z.-C. Lv, U. Schneider, ACS Catal. 2016, 6, 5747.
- 8
- 8aN. S. Chowdari, J. T. Suri, C. F. Barbas III, Org. Lett. 2004, 6, 2507;
- 8bM. Pouliquen, J. Blanchet, M.-C. Lasne, J. Rouden, Org. Lett. 2008, 10, 1029.
- 9
- 9aM. Marigo, A. Kjærsgaard, K. Juhl, N. Gathergood, K. A. Jørgensen, Chem. Eur. J. 2003, 9, 2359;
- 9bT. B. Poulsen, C. Alemparte, S. Saaby, M. Bella, K. A. Jørgensen, Angew. Chem. Int. Ed. 2005, 44, 2896; Angew. Chem. 2005, 117, 2956;
- 9cY. K. Kang, D. Y. Kim, J. Org. Chem. 2009, 74, 5734;
- 9dM. Hatano, T. Horibe, K. Ishihara, J. Am. Chem. Soc. 2010, 132, 56;
- 9eY.-P. Lou, C.-W. Zheng, R.-M. Pan, Q.-W. Jin, G. Zhao, Z. Li, Org. Lett. 2015, 17, 688;
- 9fB. M. Trost, T. Saget, C.-I. Hung, J. Am. Chem. Soc. 2016, 138, 3659.
- 10
- 10aL. Zhang, N. Fu, S. Luo, Acc. Chem. Res. 2015, 48, 986;
- 10bC. Xu, L. Zhang, S. Luo, Angew. Chem. Int. Ed. 2014, 53, 4149; Angew. Chem. 2014, 126, 4233;
- 10cL. Zhang, C. Xu, X. Mi, S. Luo, Chem. Asian J. 2014, 9, 3565;
- 10dY. You, L. Zhang, S. Luo, Chem. Sci. 2017, 8, 621.
- 11
- 11aI. Ibrahem, J. Casas, A. Córdova, Angew. Chem. Int. Ed. 2004, 43, 6528; Angew. Chem. 2004, 116, 6690;
- 11bX. Lian, L. Lin, K. Fu, B. Ma, X. Liu, X. Feng, Chem. Sci. 2017, 8, 1238;
- 11cY. Chi, S. H. Gellman, J. Am. Chem. Soc. 2006, 128, 6804;
- 11dI. Ibrahem, G.-L. Zhao, A. Córdova, Chem. Eur. J. 2007, 13, 683.
- 12Y. Numajiri, B. P. Pritchett, K. Chiyoda, B. M. Stoltz, J. Am. Chem. Soc. 2015, 137, 1040.
- 13
- 13aA review: B. Eftekhari-Sis, M. Zirak, Chem. Rev. 2017, 117, 8326. For selected examples, see:
- 13bT. Kano, Y. Yamaguchi, O. Tokuda, K. Maruoka, J. Am. Chem. Soc. 2005, 127, 16408;
- 13cH. Zhang, S. Mitsumori, N. Utsumi, M. Imai, N. Garcia-Delgado, M. Mifsud, K. Albertshofer, P. H.-Y. Cheong, K. N. Houk, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2008, 130, 875;
- 13dB. T. Hahn, R. Fröhlich, K. Harms, F. Glorius, Angew. Chem. Int. Ed. 2008, 47, 9985; Angew. Chem. 2008, 120, 10134;
- 13eD. A. Yalalov, S. B. Tsogoeva, T. E. Shubina, I. M. Martynova, T. Clark, Angew. Chem. Int. Ed. 2008, 47, 6624; Angew. Chem. 2008, 120, 6726.