A Pyrene-Linked Cavity within a β-Barrel Protein Promotes an Asymmetric Diels–Alder Reaction
Dr. Tomoki Himiyama
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorNaomasa Taniguchi
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorShunsuke Kato
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Dr. Akira Onoda
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Takashi Hayashi
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorDr. Tomoki Himiyama
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorNaomasa Taniguchi
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorShunsuke Kato
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Dr. Akira Onoda
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Takashi Hayashi
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorGraphical Abstract
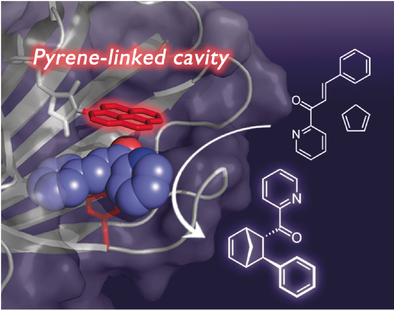
Selectivity by the barrel-load: A reaction cavity with a tethered polycyclic pyrene moiety, which acts as a platform to provide aromatic interactions, is constructed within the rigid scaffold of the β-barrel nitrobindin protein. An asymmetric Diels–Alder reaction between azachalcone and cyclopentadiene proceeds within the reaction cavity of the pyrene-linked nitrobindin with high stereoselectivity.
Abstract
A unique π-expanded reaction cavity tethering a polycyclic moiety which provides a platform for substrate binding was constructed within the robust β-barrel structure of nitrobindin (NB). NB variants with cavities of different sizes and shapes are coupled with N-(1-pyrenyl)maleimide (Pyr) to prepare a series of NB-Pyr conjugates. The orientation of the pyrene moiety is fixed within the cavity by the coupling reaction. The fluorescent quenching analysis of NB-Pyr indicates that azachalcone (aza), which is a dienophile for a Diels–Alder (DA) reaction, is efficiently incorporated within the pyrene-linked reaction cavity by the aromatic interaction. The DA reaction between aza and cyclopentadiene proceeds within the reaction cavity of NB-Pyr in the presence of CuII ion in high yield and high enantio- and regioselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201704524-sup-0001-misc_information.pdf583.2 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. Fiedler, D. H. Leung, R. G. Bergman, K. N. Raymond, Acc. Chem. Res. 2005, 38, 349–358.
- 2M. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. Int. Ed. 2009, 48, 3418–3438; Angew. Chem. 2009, 121, 3470–3490.
- 3C. R. Kennedy, S. Lin, E. N. Jacobsen, Angew. Chem. Int. Ed. 2016, 55, 12596–12624; Angew. Chem. 2016, 128, 12784–12814.
- 4L. Liu, Y. Cotelle, A.-J. Avestro, N. Sakai, S. Matile, J. Am. Chem. Soc. 2016, 138, 7876–7879.
- 5Y. Cotelle, S. Benz, A.-J. Avestro, T. R. Ward, N. Sakai, S. Matile, Angew. Chem. Int. Ed. 2016, 55, 4275–4279; Angew. Chem. 2016, 128, 4347–4351.
- 6R. R. Knowles, E. N. Jacobsen, Proc. Natl. Acad. Sci. USA 2010, 107, 20678–20685.
- 7Y. Zhao, S. Benz, N. Sakai, S. Matile, Chem. Sci. 2015, 6, 6219–6223.
- 8K. U. Wendt, G. E. Schulz, E. J. Corey, D. R. Liu, Angew. Chem. Int. Ed. 2000, 39, 2812–2833;
10.1002/1521-3773(20000818)39:16<2812::AID-ANIE2812>3.0.CO;2-# CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2930–2952.
- 9J. A. Faraldos, A. K. Antonczak, V. González, R. Fullerton, E. M. Tippmann, R. K. Allemann, J. Am. Chem. Soc. 2011, 133, 13906–13909.
- 10Y. Cotelle, V. Lebrun, N. Sakai, T. R. Ward, S. Matile, ACS Cent. Sci. 2016, 2, 388–393.
- 11J. C. Lewis, ACS Catal. 2013, 3, 2954–2975.
- 12T. R. Ward, M. R. Ringenberg, Chem. Commun. 2011, 47, 8470–8476.
- 13F. Rosati, G. Roelfes, ChemCatChem 2010, 2, 916–927.
- 14A. Chatterjee, H. Mallin, J. Klehr, J. Vallapurackal, A. D. Finke, L. Vera, M. Marsh, T. R. Ward, Chem. Sci. 2016, 7, 673–677.
- 15T. K. Hyster, L. Knörr, T. R. Ward, T. Rovis, Science 2012, 338, 500–503.
- 16V. Köhler, Y. M. Wilson, M. Dürrenberger, D. Ghislieri, E. Churakova, T. Quinto, L. Knörr, D. Häussinger, F. Hollmann, N. J. Turner, T. R. Ward, Nat. Chem. 2013, 5, 93–99.
- 17J. Bos, W. R. Browne, A. J. M. Driessen, G. Roelfes, J. Am. Chem. Soc. 2015, 137, 9796–9799.
- 18M. Bordeaux, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2015, 54, 1975–1975; Angew. Chem. 2015, 127, 1997–1997.
- 19K. Oohora, Y. Kihira, E. Mizohata, T. Inoue, T. Hayashi, J. Am. Chem. Soc. 2013, 135, 17282–17285.
- 20T. K. Ronson, A. B. League, L. Gagliardi, C. J. Cramer, J. R. Nitschke, J. Am. Chem. Soc. 2014, 136, 15615–15624.
- 21Z. Xu, N. J. Singh, J. Lim, J. Pan, H. N. Kim, S. Park, K. S. Kim, J. Yoon, J. Am. Chem. Soc. 2009, 131, 15528–15533.
- 22L. Zheng, S. Sonzini, M. Ambarwati, E. Rosta, O. A. Scherman, A. Herrmann, Angew. Chem. Int. Ed. 2015, 54, 13007–13011; Angew. Chem. 2015, 127, 13199–13203.
- 23T. Himiyama, D. F. Sauer, A. Onoda, T. P. Spaniol, J. Okuda, T. Hayashi, J. Inorg. Biochem. 2016, 158, 55–61.
- 24A. Onoda, Y. Kihara, K. Fukumoto, Y. Sano, T. Hayashi, ACS Catal. 2014, 4, 2645–2648.
- 25D. F. Sauer, T. Himiyama, K. Tachikawa, K. Fukumoto, A. Onoda, E. Mizohata, T. Inoue, M. Bocola, U. Schwaneberg, T. Hayashi, J. Okuda, ACS Catal. 2015, 5, 7519–7522.
- 26K. Fukumoto, A. Onoda, E. Mizohata, M. Bocola, T. Inoue, U. Schwaneberg, T. Hayashi, ChemCatChem 2014, 6, 1229–1235.
- 27A. Onoda, K. Fukumoto, M. Arlt, M. Bocola, U. Schwaneberg, T. Hayashi, Chem. Commun. 2012, 48, 9756–9758.
- 28C. Zhang, P. Srivastava, K. Ellis-Guardiola, J. C. Lewis, Tetrahedron 2014, 70, 4245–4249.
- 29E. Krieger, T. Darden, S. Nabuurs, A. Finkelstein, G. Vriend, Proteins Struct. Funct. Bioinf. 2004, 57, 678–683.
- 30S. Reymond, J. Cossy, Chem. Rev. 2008, 108, 5359–5406.
- 31S. Otto, G. Boccaletti, J. B. F. N. Engberts, J. Am. Chem. Soc. 1998, 120, 4238–4239.
- 32M. T. Reetz, N. Jiao, Angew. Chem. Int. Ed. 2006, 45, 2416–2419; Angew. Chem. 2006, 118, 2476–2479.
- 33J. Bos, F. Fusetti, A. J. M. Driessen, G. Roelfes, Angew. Chem. Int. Ed. 2012, 51, 7472–7475; Angew. Chem. 2012, 124, 7590–7593.
- 34P. J. Deuss, G. Popa, A. M. Z. Slawin, W. Laan, P. C. J. Kamer, ChemCatChem 2013, 5, 1184–1191.
- 35W. Ghattas, L. Cotchico-Alonso, J.-D. Maréchal, A. Urvoas, M. Rousseau, J.-P. Mahy, R. Ricoux, ChemBioChem 2016, 17, 433–440.
- 36H. Osseili, D. F. Sauer, K. Beckerle, M. Arlt, T. Himiyama, T. Polen, A. Onoda, U. Schwaneberg, T. Hayashi, J. Okuda, Beilstein J. Org. Chem. 2016, 12, 1314–1321.
- 37Y. Lu, Y. Zhou, L. Lin, H. Zheng, K. Fu, X. Liu, X. Feng, Chem. Commun. 2016, 52, 8255–8258.