Stacking and Electrostatic Interactions Drive the Stereoselectivity of Silylium-Ion Asymmetric Counteranion-Directed Catalysis
Trevor J. Seguin
Department of Chemistry, Texas A&M University, College Station, TX, 77842 USA
Search for more papers by this authorCorresponding Author
Prof. Steven E. Wheeler
Department of Chemistry, Texas A&M University, College Station, TX, 77842 USA
Search for more papers by this authorTrevor J. Seguin
Department of Chemistry, Texas A&M University, College Station, TX, 77842 USA
Search for more papers by this authorCorresponding Author
Prof. Steven E. Wheeler
Department of Chemistry, Texas A&M University, College Station, TX, 77842 USA
Search for more papers by this authorGraphical Abstract
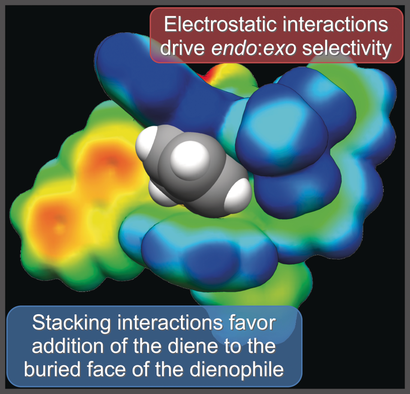
Catalysis conundrum cracked computationally: The enantioselectivity of asymmetric Lewis acid organocatalysis of the Diels–Alder cycloaddition of cyclopentadiene to cinnamates arises from stacking interactions that favor the addition of the diene to the more hindered face of the dienophile, while electrostatic interactions control the diastereoselectivity by selectively stabilizing the endo transition state.
Abstract
Computational analysis shows that the enantioselectivity of asymmetric Lewis-acid organocatalysis of the Diels–Alder cycloaddition of cyclopentadiene to cinnamates arises from stacking interactions that favor the addition of the diene to the more hindered face of the dienophile, while electrostatic interactions control the diastereoselectivity by selectively stabilizing the endo transition state. These results not only explain the stereoselectivity of these silylium-ion-ACDC reactions but should also guide the development of more effective ion-pairing asymmetric organocatalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201609095-sup-0001-misc_information.pdf683.4 KB | Supplementary |
| anie201609095-sup-0001-misc_information.txt3.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Mayer, B. List, Angew. Chem. Int. Ed. 2006, 45, 4193–4195; Angew. Chem. 2006, 118, 4299–4301;
- 1bM. Mahlau, B. List, Angew. Chem. Int. Ed. 2013, 52, 518–533; Angew. Chem. 2013, 125, 540–556.
- 2
- 2aG. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496–499;
- 2bG. L. Hamilton, T. Kanai, F. D. Toste, J. Am. Chem. Soc. 2008, 130, 14984–14986;
- 2cP. García-García, F. Lay, P. García-García, C. Rabalakos, B. List, Angew. Chem. Int. Ed. 2009, 48, 4363–4366; Angew. Chem. 2009, 121, 4427–4430;
- 2dV. Rauniyar, A. D. Lackner, G. L. Hamilton, F. D. Toste, Science 2011, 334, 1681–1684;
- 2eG. Jiang, B. List, Angew. Chem. Int. Ed. 2011, 50, 9471–9474; Angew. Chem. 2011, 123, 9643–9646;
- 2fR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603–614;
- 2gM. Mahlau, P. Garcia-Garcia, B. List, Chem. Eur. J. 2012, 18, 16283–16287;
- 2hK. Brak, E. N. Jacobsen, Angew. Chem. Int. Ed. 2013, 52, 534–561; Angew. Chem. 2013, 125, 558–588;
- 2iA. J. Neel, J. P. Hehn, P. F. Tripet, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 14044–14047;
- 2jW. Chen, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 2068–2071;
- 2kQ. Wang, M. Leutzsch, M. van Gemmeren, B. List, J. Am. Chem. Soc. 2013, 135, 15334–15337;
- 2lX. Yang, R. J. Phipps, F. D. Toste, J. Am. Chem. Soc. 2014, 136, 5225–5228;
- 2mP.-S. Wang, H.-C. Lin, Y.-J. Zhai, Z.-Y. Han, L.-Z. Gong, Angew. Chem. Int. Ed. 2014, 53, 12218–12221; Angew. Chem. 2014, 126, 12414–12417;
- 2nZ. Zhang, H. Y. Bae, J. Guin, C. Rabalakos, M. van Gemmeren, M. Leutzsch, M. Klussmann, B. List, Nat. Commun. 2016, 7, 12478;
- 2oK. Hiramatsu, T. Honjo, V. Rauniyar, F. D. Toste, ACS Catal. 2016, 6, 151–154.
- 3
- 3aK. N. Houk, P. H.-Y. Cheong, Nature 2008, 455, 309–313;
- 3bP. H.-Y. Cheong, C. Y. Legault, J. M. Um, N. Çelebi-Olçüm, K. N. Houk, Chem. Rev. 2011, 111, 5042–5137;
- 3cR. B. Sunoj, Acc. Chem. Res. 2016, 49, 1019–1028;
- 3dD. M. Walden, O. M. Ogba, R. C. Johnston, P. H.-Y. Cheong, Acc. Chem. Res. 2016, 49, 1279–1291;
- 3eS. E. Wheeler, T. J. Seguin, Y. Guan, A. C. Doney, Acc. Chem. Res. 2016, 49, 1061–1069;
- 3fY. Lam, M. N. Grayson, M. C. Holland, A. Simon, K. N. Houk, Acc. Chem. Res. 2016, 49, 750–762;
- 3gK. S. Halskov, B. S. Donslund, B. M. Paz, K. A. Jørgensen, Acc. Chem. Res. 2016, 49, 974–986;
- 3hJ. P. Reid, L. Simón, J. M. Goodman, Acc. Chem. Res. 2016, 49, 1029–1041;
- 3iQ. Peng, R. S. Paton, Acc. Chem. Res. 2016, 49, 1042–1051.
- 4T. Lu, S. E. Wheeler, Science 2015, 347, 719–720.
- 5A. Milo, A. J. Neel, F. D. Toste, M. S. Sigman, Science 2015, 347, 737–743.
- 6T. Gatzenmeier, M. van Gemmeren, Y. Xie, D. Höfler, M. Leutzsch, B. List, Science 2016, 351, 949–952.
- 7B. Mathieu, L. de Fays, L. Ghosez, Tetrahedron Lett. 2000, 41, 9561–9564.
- 8
- 8aS. Miertus, E. Scrocco, J. Tomasi, Chem. Phys. 1981, 55, 117–129;
- 8bJ. Tomasi, B. Mennucci, R. Cammi, Chem. Rev. 2005, 105, 2999–3093;
- 8cY. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241.
- 9S. J. Connon, Angew. Chem. Int. Ed. 2006, 45, 3909–3912; Angew. Chem. 2006, 118, 4013–4016.
- 10S. E. Wheeler, K. N. Houk, J. Chem. Theory Comput. 2010, 6, 395–404.
- 11S. Grimme, Chem. Eur. J. 2012, 18, 9955–9964.
- 12R. C. Johnston, P. H.-Y. Cheong, Org. Biomol. Chem. 2013, 11, 5057–5064.
- 13TS structures are named based on the corresponding entry in Table 1, whether the TS leads to the favored or disfavored (denoted by a prime) enantiomer, and whether the TS leads to the endo or exo adduct. For instance, TS(3)endo is the lower-lying endo TS for entry 3, whereas TS(3′)endo is the most favourable endo TS leading to the disfavored enantiomer for this entry.
- 14
- 14aT. J. Seguin, T. Lu, S. E. Wheeler, Org. Lett. 2015, 17, 3066–3069;
- 14bD. H. Ess, K. N. Houk, J. Am. Chem. Soc. 2007, 129, 10646–10647;
- 14cF. M. Bickelhaupt, W. J. van Zeist, Org. Biomol. Chem. 2010, 8, 3118–3127.
- 15J. P. Wagner, P. R. Schreiner, Angew. Chem. Int. Ed. 2015, 54, 12274–12296; Angew. Chem. 2015, 127, 12446–12471.
- 16Et2O is less polarizable than toluene and, therefore, will engage in weaker dispersion interactions with the exposed CP in transition states such as TS(3′)endo. The inability of continuum solvent models to fully account for such interactions likely contributes to the overestimation of stereoselectivities of these reactions.
- 17
- 17aR. C. Johnston, D. T. Cohen, C. C. Eichman, K. A. Scheidt, P. H.-Y. Cheong, Chem. Sci. 2014, 5, 1974–1982;
- 17bT. J. Seguin, S. E. Wheeler, ACS Catal. 2016, 6, 2681–2688;
- 17cA. C. Doney, B. R. Rooks, T. Lu, S. E. Wheeler, ACS Catal. 2016, 6, 7948–7955.