A PCP Pincer Ligand for Coordination Polymers with Versatile Chemical Reactivity: Selective Activation of CO2 Gas over CO Gas in the Solid State
Junpeng He
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorNolan W. Waggoner
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorSamuel G. Dunning
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorDr. Alexander Steiner
Department of Chemistry, University of Liverpool, Crown St., Liverpool, L69 7ZD UK
Search for more papers by this authorDr. Vincent M. Lynch
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorCorresponding Author
Prof. Simon M. Humphrey
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorJunpeng He
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorNolan W. Waggoner
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorSamuel G. Dunning
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorDr. Alexander Steiner
Department of Chemistry, University of Liverpool, Crown St., Liverpool, L69 7ZD UK
Search for more papers by this authorDr. Vincent M. Lynch
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorCorresponding Author
Prof. Simon M. Humphrey
Department of Chemistry, The University of Texas at Austin, NHB 6.336, 100 E. 24th St. Stop A1590, Austin, TX, 78712 USA
Search for more papers by this authorGraphical Abstract
Abstract
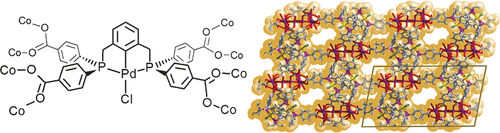
A tetra(carboxylated) PCP pincer ligand has been synthesized as a building block for porous coordination polymers (PCPs). The air- and moisture-stable PCP metalloligands are rigid tetratopic linkers that are geometrically akin to ligands used in the synthesis of robust metal–organic frameworks (MOFs). Here, the design principle is demonstrated by cyclometalation with PdIICl and subsequent use of the metalloligand to prepare a crystalline 3D MOF by direct reaction with CoII ions and structural resolution by single crystal X-ray diffraction. The Pd−Cl groups inside the pores are accessible to post-synthetic modifications that facilitate chemical reactions previously unobserved in MOFs: a Pd−CH3 activated material undergoes rapid insertion of CO2 gas to give Pd−OC(O)CH3 at 1 atm and 298 K. However, since the material is highly selective for the adsorption of CO2 over CO, a Pd−N3 modified version resists CO insertion under the same conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201604730-sup-0001-misc_information.pdf4.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Horike, D. Umeyama, S. Kitagawa, Acc. Chem. Res. 2013, 46, 2376–2384;
- 1bJ. L. C. Rowsell, J. Eckert, O. M. Yaghi, J. Am. Chem. Soc. 2005, 127, 14904–14910;
- 1cN. Yanai, T. Uemura, M. Inoue, R. Matsuda, T. Fukushima, M. Tsujimoto, S. Isoda, S. Kitagawa, J. Am. Chem. Soc. 2012, 134, 4501–4504;
- 1dR. Krishna, J. M. van Baten, J. Phys. Chem. C 2012, 116, 23556–23568.
- 2
- 2aD. Jiang, T. Mallat, F. Krumeich, A. Baiker, J. Catal. 2008, 257, 390–395;
- 2bL. Chen, S. Rangan, J. Li, H. Jiang, Y. Li, Green Chem. 2014, 16, 3978–3985;
- 2cZ. Wang, J. Liu, H. K. Arslan, S. Grosjean, T. Hagendorn, H. Gliemann, S. Bräse, C. Wöll, Langmuir 2013, 29, 15958–15964.
- 3
- 3aM. Sabo, A. Henschel, H. Fröde, E. Klemm, S. Kaskel, J. Mater. Chem. 2007, 17, 3827–3832;
- 3bK. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. Bae, J. R. Long, Chem. Rev. 2012, 112, 724–781;
- 3cS. S. Kaye, A. Dailly, O. M. Yaghi, J. R. Long, J. Am. Chem. Soc. 2007, 129, 14176–14177;
- 3dQ. Yang, C. Zong, J. Phys. Chem. B 2006, 110, 655–658.
- 4
- 4aL. J. McCormick, S. G. Duyker, A. W. Thornton, C. S. Hawes, M. R. Hill, V. K. Peterson, S. R. Batten, D. R. Turner, Chem. Mater. 2014, 26, 4640–4646;
- 4bS. Yang, G. S. B. Martin, J. J. Titman, A. J. Blake, D. R. Allan, N. R. Champness, M. Schröder, Inorg. Chem. 2011, 50, 9374–9384;
- 4cB. Chen, X. Zhao, A. Putkham, K. Hong, E. B. Lobkovsky, E. J. Hurtado, A. J. Fletcher, K. M. Thomas, J. Am. Chem. Soc. 2008, 130, 6411–6423;
- 4dT. K. Prasad, D. H. Hong, M. P. Suh, Chem. Eur. J. 2010, 16, 14043–14050.
- 5
- 5aA. Karmakar, A. V. Desai, S. K. Ghosh, Coord. Chem. Rev. 2016, 307, 313–341;
- 5bM. Owczarek, P. Szklarz, R. Jakubas, A. Miniewicz, Dalton Trans. 2012, 41, 7285–7294;
- 5cD. Di Sante, A. Stroppa, P. Jain, S. Picozzi, J. Am. Chem. Soc. 2013, 135, 18126–18130;
- 5dR. Shang, G. Xu, Z. Wang, S. Gao, Chem. Eur. J. 2014, 20, 1146–1158.
- 6
- 6aT. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong, J. R. Long, J. Am. Chem. Soc. 2012, 134, 7056–7065;
- 6bA. M. Fracaroli, H. Furukawa, M. Suzuki, M. Dodd, S. Okajima, F. Gándara, J. A. Reimer, O. M. Yaghi, J. Am. Chem. Soc. 2014, 136, 8863–8866;
- 6cJ. A. Mason, T. M. McDonald, T. Bae, J. E. Bachman, K. Sumida, J. J. Dutton, S. S. Kaye, J. R. Long, J. Am. Chem. Soc. 2015, 137, 4787–4803;
- 6dS. Choi, T. Watanabe, T. Bae, D. S. Sholl, C. W. Jones, J. Phys. Chem. Lett. 2012, 3, 1136–1141.
- 7
- 7aA. J. Blake, N. R. Champness, T. L. Easun, D. R. Allan, H. Nowell, M. W. George, J. Jia, X.-Z. Sun, Nat. Chem. 2010, 2, 688–694;
- 7bK. Manna, T. Zhang, F. X. Greene, W. Lin, J. Am. Chem. Soc. 2015, 137, 2665–2673;
- 7cK. Ikemoto, Y. Inokuma, K. Rissanen, M. Fujita, J. Am. Chem. Soc. 2014, 136, 6892–6895;
- 7dJ. Lee, O. K. Farha, J. Roberts, K. A. Schidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450–1459;
- 7eK. K. Tanabe, S. M. Cohen, Chem. Soc. Rev. 2011, 40, 498–519;
- 7fM. Yoon, R. Srirambalaji, K. Kim, Chem. Rev. 2012, 112, 1196–1231;
- 7gC. Wu, A. Hu, L. Zhang, W. Lin, J. Am. Chem. Soc. 2005, 127, 8940–8941;
- 7hS. A. Burgess, A. Kassie, S. A. Baranowski, K. J. Fritzsching, K. Schmidt-Rohr, C. M. Brown, C. R. Wade, J. Am. Chem. Soc. 2016, 138, 1780–1783.
- 8
- 8aW. Gao, L. Wojtas, S. Ma, Chem. Commun. 2014, 50, 5316–5318;
- 8bC. Wang, Z. Xie, K. E. deKrafft, W. Lin, J. Am. Chem. Soc. 2011, 133, 13445–13454;
- 8cH. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai, Q. Xu, J. Am. Chem. Soc. 2009, 131, 11302–11303;
- 8dJ. Liu, Y. Wang, A. I. Benin, P. Jakubczak, R. R. Willis, M. D. LeVan, Langmuir 2010, 26, 14301–14307.
- 9
- 9aL. Zhou, X. Lin, T. Huang, A. Yu, Electrochim. Acta 2014, 116, 210–216;
- 9bJ. Graciani, K. Mudiyanselage, F. Xu, A. E. Baber, J. Evans, S. D. Senanayake, D. J. Stacchiola, P. Liu, J. Hrbek, J. F. Sanz, J. A. Rodriguez, Science 2014, 345, 546–550;
- 9cN. P. Wickramaratne, J. Xu, M. Wang, L. Zhu, L. Dai, M. Jaroniec, Chem. Mater. 2014, 26, 2820–2828;
- 9dK. T. Cho, S. B. Lee, J. W. Lee, J. Phys. Chem. C 2014, 118, 9357–9367.
- 10
- 10aB. M. Hoffman, D. Lukoyanov, Z. Yang, D. R. Dean, L. C. Seefeldt, Chem. Rev. 2014, 114, 4041–4062;
- 10bT. M. Figg, P. L. Holland, T. R. Cundari, Inorg. Chem. 2012, 51, 7546–7550;
- 10cQ. Cui, D. G. Musaev, M. Svensson, S. Sieber, K. Morokuma, J. Am. Chem. Soc. 1995, 117, 12366–12367.
- 11
- 11aS. Park, Y. Chun, S. J. Cho, S. Lee, K. S. Kim, J. Chem. Theory Comput. 2012, 8, 1983–1988;
- 11bA. M. Chapman, M. F. Haddow, D. F. Wass, J. Am. Chem. Soc. 2011, 133, 18463–18478;
- 11cS. N. Riduan, Y. Zhang, J. Y. Ying, Angew. Chem. Int. Ed. 2009, 48, 3322–3325; Angew. Chem. 2009, 121, 3372–3375.
- 12
- 12aX. Yu, S. M. Cohen, Chem. Commun. 2015, 51, 9880–9883;
- 12bP. V. Dau, M. Kim, S. M. Cohen, Chem. Sci. 2013, 4, 601–605;
- 12cW. M. Bloch, A. Burgun, C. J. Coghlan, R. Lee, M. L. Coote, C. J. Doonan, C. J. Sumby, Nat. Chem. 2014, 6, 906–912.
- 13
- 13aW. M. Bloch, N. R. Champness, C. J. Doonan, Angew. Chem. Int. Ed. 2015, 54, 12860–12867; Angew. Chem. 2015, 127, 13052–13059;
- 13bF. Wang, Y. Tan, H. Yang, Y. Kang, J. Zhang, Chem. Commun. 2012, 48, 4842–4844;
- 13cL. Zhang, Y. Qin, Z. Li, Q. Lin, J. Cheng, J. Zhang, Y. Yao, Inorg. Chem. 2008, 47, 8286–8293.
- 14
- 14aK. W. Anderson, S. L. Buchwald, Angew. Chem. Int. Ed. 2005, 44, 6173–6177; Angew. Chem. 2005, 117, 6329–6333;
- 14bD. E. Bergbreiter, P. L. Osburn, A. Wilson, E. M. Sink, J. Am. Chem. Soc. 2000, 122, 9058–9064.
- 15A. M. Bohnsack, I. A. Ibarra, V. I. Bakhmutov, V. M. Lynch, S. M. Humphrey, J. Am. Chem. Soc. 2013, 135, 16038–16041.
- 16E. Tsivion, J. R. Long, M. Head-Gordon, J. Am. Chem. Soc. 2014, 136, 17827–17835.
- 17
- 17aW. Leis, H. A. Mayer, W. C. Kaska, Coord. Chem. Rev. 2008, 252, 1787–1797;
- 17bC. M. Jensen, Chem. Commun. 1999, 2443–2449;
- 17cD. Benito-Garagorri, K. Kirchner, Acc. Chem. Res. 2008, 41, 201–213.
- 18J. J. M. de Pater, C. E. P. Maljaars, E. de Wolf, M. Lutz, A. L. Spek, B. Deelman, C. J. Elsevier, G. van Koten, Organometallics 2005, 24, 5299–5310.
- 19See for example:
- 19aO. K. Farha, C. D. Malliakas, M. Kanatzidis, J. T. Hupp, J. Am. Chem. Soc. 2010, 132, 950–952;
- 19bY. Yan, M. Juríček, F.-X. Coudert, N. A. Vermeulen, S. Grunder, A. Dailly, W. Lewis, A. J. Blake, J. F. Stoddart, M. Schroder, J. Am. Chem. Soc. 2016, 138, 3371–3381.
- 20M. C. Denney, N. A. Smythe, K. L. Cetto, R. A. Kemp, K. I. Goldberg, J. Am. Chem. Soc. 2006, 128, 2508–2509.
- 21H.-B. Kraatz, M. E. van der Boom, Y. Ben-David, D. Milstein, Isr. J. Chem. 2001, 41, 163–171.
- 22J. R. Durig, R. Layton, D. W. Sink, B. R. Mitchell, Spectrochim. Acta 1965, 21, 1367–1378.
- 23
- 23aR. Johansson, O. F. Wendt, Dalton Trans. 2007, 488–492;
- 23bM. T. Johnson, R. Johansson, M. V. Kondrashov, G. Steyl, M. S. G. Ahlquist, A. Roodt, O. F. Wendt, Organometallics 2010, 29, 3521–3529.
- 24
- 24aT. J. Schmeier, N. Hazari, C. D. Incarvito, J. A. Raskatov, Chem. Commun. 2011, 47, 1824–1826;
- 24bR. Johansson, M. Jarenmark, O. F. Wendt, Organometallics 2005, 24, 4500–4502.
- 25
- 25aB. Ding, Z. Zhang, Y. Liu, M. Sugiya, T. Imamoto, W. Zhang, Org. Lett. 2013, 15, 3690–3693;
- 25bX.-Y. Yang, J. H. Gan, Y. Li, S. A. Pullarkat, P.-H. Leung, Dalton Trans. 2015, 44, 1258–1263.
- 26H. J. Lee, S. H. Lee, H. C. Kim, Y.-E. Lee, S. Park, J. Organomet. Chem. 2012, 717, 164–171.
- 27J.-R. Li, J. Sculley, H.-C. Zhou, Chem. Rev. 2012, 112, 869–932.
- 28CCDC 1479781 (6) and 1479780 (PCM-36) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.