Redox Switching of Orthoquinone-Containing Aromatic Compounds with Hydrogen and Oxygen Gas
Kazuki Urakawa
Division of Organic Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorProf. Dr. Michinori Sumimoto
Division of Computational Chemistry, Graduate School of Science and Engineering, Yamaguchi University, 2-16-1, Tokiwadai, Ube, Yamaguchi, 755-8611 Japan
Search for more papers by this authorProf. Dr. Mitsuhiro Arisawa
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamada-oka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masaki Matsuda
Division of Physical Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hayato Ishikawa
Division of Organic Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorKazuki Urakawa
Division of Organic Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorProf. Dr. Michinori Sumimoto
Division of Computational Chemistry, Graduate School of Science and Engineering, Yamaguchi University, 2-16-1, Tokiwadai, Ube, Yamaguchi, 755-8611 Japan
Search for more papers by this authorProf. Dr. Mitsuhiro Arisawa
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamada-oka, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masaki Matsuda
Division of Physical Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hayato Ishikawa
Division of Organic Chemistry, Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto, 860-8555 Japan
Search for more papers by this authorGraphical Abstract
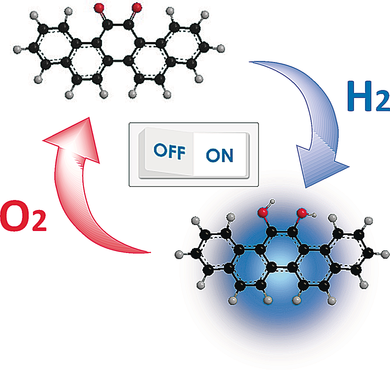
A tale with a twist: Unique redox switching of orthoquinone-containing pentacyclic aromatic compounds was observed upon exposure to H2 and O2 in the presence of a sulfur-modified gold-supported palladium nanoparticle catalyst (see picture). Switching between the orthoquinone and the corresponding hydroquinone led to a drastic change in the photoluminescence and color of the system owing to differences in the aromaticity and twist strain of the molecules.
Abstract
Unique redox switching of orthoquinone-containing pentacyclic aromatic compounds with molecular hydrogen and oxygen in the presence of a palladium nanoparticle catalyst (SAPd) is disclosed. These molecules were predicted by in silico screening before synthesis. Efficient protocols for the synthesis of orthoquinone-containing aromatic compounds by palladium-mediated homocoupling and the benzoin condensation reaction were developed. Clear switching between orthoquinone and aromatic hydroquinone compounds was observed on the basis of their photoluminescence properties. Furthermore, the twist strain of the orthoquinone moiety could induce dramatic changes in color and emission.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201601906-sup-0001-misc_information.pdf9.1 MB | Supplementary |
| anie201601906-sup-0001-movie.pptx14.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 The Physics and Chemistry of Color: The Fifteen Causes of Color (Ed.: ), Wiley, New York, 2001.
- 2 Electrochromic Materials and Devices (Eds.: ), Wiley-VCH, New York, 2015.
- 3Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liua, J. Xu, Chem. Soc. Rev. 2012, 41, 3878–3896.
- 4C. Reichardt, Chem. Rev. 1994, 94, 2319–2358.
- 5 A Theory of Halochromism (Ed.: ), København Høst, København, 1931.
- 6
- 6aC. H. Lin, J. F. Jhang, D. Y. Yang, Org. Lett. 2009, 11, 4064–4067;
- 6bC. H. Lin, J. F. Jhang, D. Y. Yang, J. Comb. Chem. 2010, 12, 119–124;
- 6cS. C. Lin, J. F. Jhang, T. J. Liou, D. Y. Yang, Dyes Pigm. 2015, 114, 259–266;
- 6dY. Miura, H. Kawai, K. Fujiwara, T. Suzuki, Chem. Lett. 2011, 40, 975–977;
- 6eG. Hennrich, H. Sonnenschein, U. Resch-Genger, J. Am. Chem. Soc. 1999, 121, 5073–5074.
- 7
- 7aT. M. Penning, M. E. Burczynski, C. F. Hung, K. D. McCoull, N. T. Palackal, L. S. Tsuruda, Chem. Res. Toxicol. 1999, 12, 1–18;
- 7bD. Schweinfurth, M. Zalibera, M. Kathan, C. Shen, M. Mazzolini, N. Trapp, J. Crassous, G. Gescheidt, F. Diederich, J. Am. Chem. Soc. 2014, 136, 13045–13052.
- 8
- 8aA. Modler-Spreitzer, R. Fritsch, A. Mannschreck, Collect. Czech. Chem. Commun. 2000, 65, 555–560;
- 8bD. Enders, O. Niemeier, Synlett 2004, 2111–2114.
- 9C. H. Lin, D. Y. Yang, Org. Lett. 2013, 15, 2802–2805.
- 10
- 10aW. Davies, B. C. Ennis, J. Chem. Soc. 1959, 915–918;
- 10bE. J. Moriconi, B. Rakoczy, W. F. O'Connor, J. Org. Chem. 1962, 27, 2772–2776.
- 11
- 11aA. D. Becke, Phys. Rev. A 1988, 38, 3098–3100;
- 11bJ. P. Perdew, Y. Wang, Phys. Rev. B 1992, 45, 13244–13249.
- 12P. von R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317–6318.
- 13
- 13aJ. R. Cheeseman, G. W. Trucks, T. A. Keith, M. J. Frisch, J. Chem. Phys. 1996, 104, 5497–5509;
- 13bK. Wolinski, J. F. Hilton, P. Pulay, J. Am. Chem. Soc. 1990, 112, 8251–8260.
- 14P. von R. Schleyer, M. Manoharan, Z. X. Wang, B. Kiran, H. Jiao, R. Puchta, N. J. R. van Eikema Hommes, Org. Lett. 2001, 3, 2465–2468.
- 15Y. Xia, Z. Liu, Q. Xiao, P. Qu, R. Ge, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2012, 51, 5714–5717; Angew. Chem. 2012, 124, 5812–5815.
- 16I. W. J. Still, R. Natividad-Preyra, F. D. Toste, Can. J. Chem. 1999, 77, 113–121.
- 17
- 17aJ. Meng, M. Gao, H. Lv, X. Zhang, Org. Lett. 2015, 17, 1842–1845;
- 17bH. Stetter, Angew. Chem. Int. Ed. Engl. 1976, 15, 639–647; Angew. Chem. 1976, 88, 695–704.
- 18
- 18aN. Hoshiya, M. Shimoda, H. Yoshikawa, Y. Yamashita, S. Shuto, M. Arisawa, J. Am. Chem. Soc. 2010, 132, 7270–7272;
- 18bN. Hoshiya, S. Shuto, M. Arisawa, Adv. Synth. Catal. 2011, 353, 743–748;
- 18cM. Al-Amin, T. Honma, N. Hoshiya, S. Shuto, M. Arisawa, Adv. Synth. Catal. 2012, 354, 1061–1068;
- 18dM. Al-Amin, M. Akimoto, T. Tameno, Y. Ohki, N. Takahashi, N. Hoshiya, S. Shuto, M. Arisawa, Green Chem. 2013, 15, 1142–1145;
- 18eM. Al-Amin, S. Arai, N. Hoshiya, T. Honma, Y. Tamenori, T. Sato, M. Yokoyama, A. Ishii, M. Takeuchi, T. Maruko, S. Shuto, M. Arisawa, J. Org. Chem. 2013, 78, 7575–7581;
- 18fM. Arisawa, T. Sato, M. Al-Amin, N. Hoshiya, Y. Kogami, S. Shuto, ACS Comb. Sci. 2014, 16, 215–220;
- 18gM. Al-Amin, M. Arisawa, S. Shuto, Y. Ano, M. Tobisu, N. Chatani, Adv. Synth. Catal. 2014, 356, 1631–1637;
- 18hK. Takagi, M. Al-Amin, N. Hoshiya, J. Wouters, H. Sugimoto, Y. Shiro, H. Fukuda, S. Shuto, M. Arisawa, J. Org. Chem. 2014, 79, 6366–6371;
- 18iK. Takagi, H. Fukuda, S. Shuto, A. Otaka, M. Arisawa, Adv. Synth. Catal. 2015, 357, 2119–2124;
- 18jN. Saito, T. Taniguchi, N. Hoshiya, S. Shuto, M. Arisawa, Y. Sato, Green Chem. 2015, 17, 2358–2361;
- 18kM. Arisawa, M. Al-Amin, T. Honma, Y. Tamenori, S. Arai, N. Hoshiya, T. Sato, M. Yokoyama, A. Ishii, M. Takeguchi, T. Miyazaki, M. Takeuchi, T. Maruko, S. Shuto, RSC Adv. 2015, 5, 676–683.
- 19G. M. Badger, P. R. Jefferies, R. W. L. Kimber, J. Chem. Soc. 1957, 1837–1841.