Deriving Structural Information from Experimentally Measured Data on Biomolecules
Corresponding Author
Wilfred F. van Gunsteren
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorJane R. Allison
Centre for Theor. Chem. and Phys. & Institute of Natural and Mathematical Sciences, Massey Univ., Auckland, New Zealand
Biomolecular Interaction Centre, University of Canterbury, Christchurch, New Zealand
Maurice Wilkins Centre for Molecular Biodiscovery, New Zealand
Search for more papers by this authorXavier Daura
Institute of Biotechnology and Biomedicine, Universitat Autònoma de Barcelona (UAB), 08193 Barcelona, Spain
Catalan Institution for Research and Advanced Studies (ICREA), 08010 Barcelona, Spain
Search for more papers by this authorJožica Dolenc
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorNiels Hansen
Institute of Thermodynamics and Thermal Process Engineering, University of Stuttgart, Pfaffenwaldring 9, 70569 Stuttgart, Germany
Search for more papers by this authorAlan E. Mark
School of Chemistry and Molecular Biosciences, University of Queensland, St. Lucia, QLD 4072 Australia
Search for more papers by this authorChris Oostenbrink
Institute of Molecular Modeling and Simulation, University of Natural Resources and Life Sciences, Vienna, Austria
Search for more papers by this authorVictor H. Rusu
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorLorna J. Smith
Department of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorCorresponding Author
Wilfred F. van Gunsteren
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorJane R. Allison
Centre for Theor. Chem. and Phys. & Institute of Natural and Mathematical Sciences, Massey Univ., Auckland, New Zealand
Biomolecular Interaction Centre, University of Canterbury, Christchurch, New Zealand
Maurice Wilkins Centre for Molecular Biodiscovery, New Zealand
Search for more papers by this authorXavier Daura
Institute of Biotechnology and Biomedicine, Universitat Autònoma de Barcelona (UAB), 08193 Barcelona, Spain
Catalan Institution for Research and Advanced Studies (ICREA), 08010 Barcelona, Spain
Search for more papers by this authorJožica Dolenc
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorNiels Hansen
Institute of Thermodynamics and Thermal Process Engineering, University of Stuttgart, Pfaffenwaldring 9, 70569 Stuttgart, Germany
Search for more papers by this authorAlan E. Mark
School of Chemistry and Molecular Biosciences, University of Queensland, St. Lucia, QLD 4072 Australia
Search for more papers by this authorChris Oostenbrink
Institute of Molecular Modeling and Simulation, University of Natural Resources and Life Sciences, Vienna, Austria
Search for more papers by this authorVictor H. Rusu
Laboratory of Physical Chemistry, Swiss Federal Institute of Technology, ETH, 8093 Zurich, Switzerland
Search for more papers by this authorLorna J. Smith
Department of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR UK
Search for more papers by this authorGraphical Abstract
Making the right choice: Deriving structural information from experimentally measured data is a process beset with theoretical and practical problems. The effects of the various assumptions and approximations involved in the process of biomolecular structure determination are discussed and a list of choices to be avoided is provided.
Abstract
During the past half century, the number and accuracy of experimental techniques that can deliver values of observables for biomolecular systems have been steadily increasing. The conversion of a measured value Qexp of an observable quantity Q into structural information is, however, a task beset with theoretical and practical problems: 1) insufficient or inaccurate values of Qexp, 2) inaccuracies in the function 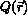 used to relate the quantity Q to structure
used to relate the quantity Q to structure 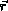 , 3) how to account for the averaging inherent in the measurement of Qexp, 4) how to handle the possible multiple-valuedness of the inverse
, 3) how to account for the averaging inherent in the measurement of Qexp, 4) how to handle the possible multiple-valuedness of the inverse 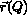 of the function
of the function 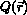 , to mention a few. These apply to a variety of observable quantities Q and measurement techniques such as X-ray and neutron diffraction, small-angle and wide-angle X-ray scattering, free-electron laser imaging, cryo-electron microscopy, nuclear magnetic resonance, electron paramagnetic resonance, infrared and Raman spectroscopy, circular dichroism, Förster resonance energy transfer, atomic force microscopy and ion-mobility mass spectrometry. The process of deriving structural information from measured data is reviewed with an eye to non-experts and newcomers in the field using examples from the literature of the effect of the various choices and approximations involved in the process. A list of choices to be avoided is provided.
, to mention a few. These apply to a variety of observable quantities Q and measurement techniques such as X-ray and neutron diffraction, small-angle and wide-angle X-ray scattering, free-electron laser imaging, cryo-electron microscopy, nuclear magnetic resonance, electron paramagnetic resonance, infrared and Raman spectroscopy, circular dichroism, Förster resonance energy transfer, atomic force microscopy and ion-mobility mass spectrometry. The process of deriving structural information from measured data is reviewed with an eye to non-experts and newcomers in the field using examples from the literature of the effect of the various choices and approximations involved in the process. A list of choices to be avoided is provided.
References
- 1J. C. Kendrew, G. Bodo, H. M. Dintzis, R. G. Parrish, H. Wyckoff, D. C. Phillips, Nature 1958, 181, 662–666.
- 2C. C. F. Blake, D. F. Koenig, G. A. Mair, A. C. T. North, D. C. Phillips, V. R. Sarma, Nature 1965, 206, 757–761.
- 3J. Drenth, C. M. Enzing, K. H. Kalk, J. C. A. Vessies, Nature 1976, 264, 373–377.
- 4A. Wlodawer, J. Walter, R. Huber, L. Sjölin, J. Mol. Biol. 1984, 180, 301–329.
- 5D. I. Svergun, M. H. J. Koch, Rep. Prog. Phys. 2003, 66, 1735–1782.
- 6K. J. Gaffney, H. N. Chapman, Science 2007, 316, 1444–1448.
- 7M. Walczak, H. Grubmüller, Phys. Rev. E 2014, 90, 022714.
- 8X.-C. Bai, G. McMullan, S. H. W. Scheres, Trends Biochem. Sci. 2015, 40, 49–57.
- 9R. R. Ernst, G. Bodenhausen, A. Wokaun, Principles of Nuclear Magnetic Resonance in One and Two Dimensions, Clarendon, Oxford, 1990.
10.1093/oso/9780198556473.001.0001 Google Scholar
- 10K. D. Berndt, P. Güntert, L. P. M. Orbons, K. Wüthrich, J. Mol. Biol. 1992, 227, 757–775.
- 11C. C. Jao, B. G. Hegde, J. Chen, I. S. Haworth, R. Langen, Proc. Natl. Acad. Sci. USA 2008, 105, 19666–19671.
- 12S. Bagchi, S. G. Boxer, M. D. Fayer, J. Phys. Chem. B 2012, 116, 4034–4042.
- 13Y. Hong, L. Meng, S. Chen, C. W. T. Leung, L.-T. Da, M. Faisal, D.-A. Silva, J. Liu, J. W. Y. Lam, X. Huang, B. Z. Tang, J. Am. Chem. Soc. 2012, 134, 1680–1689.
- 14C. A. E. Hauser, R. Deng, A. Mishra, Y. Loo, U. Khoe, F. Zhuang, D. W. Cheong, A. Accardo, M. B. Sullivan, C. Riekel, J. Y. Ying, U. A. Hauser, Proc. Natl. Acad. Sci. USA 2011, 108, 1361–1366.
- 15M. Hoefling, N. Lima, D. Haenni, C. A. M. Seidel, B. Schuler, H. Grubmüller, PLoS One 2011, 6, e 19791.
- 16M. M. Reif, C. Oostenbrink, J. Comput. Chem. 2014, 35, 2319–2332.
- 17A. T. Brunger, P. Strop, M. Vrljic, S. Chu, K. R. Weninger, J. Struct. Biol. 2011, 173, 497–505.
- 18T. Ando, Nanotechnology 2012, 23, 062001.
- 19Z. Hall, A. Politis, M. F. Bush, L. J. Smith, C. V. Robinson, J. Am. Chem. Soc. 2012, 134, 3429–3438.
- 20J. Drenth, Principles of Protein X-ray Crystallography, Springer, New York, 1994.
10.1007/978-1-4757-2335-9 Google Scholar
- 21R. Boelens, T. M. G. Koning, R. Kaptein, J. Mol. Struct. 1988, 173, 299–311.
- 22M. Karplus, J. Chem. Phys. 1959, 30, 11–15.
- 23D. Steiner, J. R. Allison, W. F. van Gunsteren, J. Biomol. NMR 2012, 53, 223–246.
- 24B. Han, Y. Liu, S. W. Ginzinger, D. S. Wishart, J. Biomol. NMR 2011, 50, 43–57.
- 25W. F. van Gunsteren, A. M. J. J. Bonvin, X. Daura, L. J. Smith in Structure Computation and Dynamics in Protein NMR, Biol. Magnetic Resonance, Vol. 17 (Eds.: ), Plenum, New York, 1999, pp. 3–35.
- 26J. H. Lee, F. Li, A. Grishaev, A. Bax, J. Am. Chem. Soc. 2015, 137, 1432–1435.
- 27U. Stocker, D. Juchli, W. F. van Gunsteren, Mol. Simul. 2003, 29, 123–138.
- 28A. T. Brünger, X-PLOR. A System for X-ray Crystallography and NMR, Yale University Press, New Haven, 1992.
- 29W. A. Hendrickson, J. H. Konnert in Biomolecular Structure, Conformation, Function and Evolution, Vol. 1 of Diffraction and Related Studies (Ed.: ), Pergamon, Oxford, 1981, pp. 43–57.
10.1016/B978-1-4832-8364-7.50009-X Google Scholar
- 30W. F. van Gunsteren, R. Kaptein, E. R. P. Zuiderweg in Proceedings NATO/CECAM workshop on nucleic acid conformation and dynamics (Ed.: ), CECAM, Orsay, 1984, pp. 79–92.
- 31R. Kaptein, E. R. P. Zuiderweg, R. M. Scheek, R. Boelens, W. F. van Gunsteren, J. Mol. Biol. 1985, 182, 179–182.
- 32A. T. Brünger, J. Kuriyan, M. Karplus, Science 1987, 235, 458–460.
- 33M. Fujinaga, P. Gros, W. F. van Gunsteren, J. Appl. Crystallogr. 1989, 22, 1–8.
- 34G. F. Schröder, Curr. Opin. Struct. Biol. 2015, 31, 20–27.
- 35H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, P. E. Bourne, Nucleic Acids Res. 2000, 28, 235–242.
- 36W. F. van Gunsteren, J. Dolenc, A. E. Mark, Curr. Opin. Struct. Biol. 2008, 18, 149–153.
- 37A. Glättli, W. F. van Gunsteren, Angew. Chem. Int. Ed. 2004, 43, 6312–6316; Angew. Chem. 2004, 116, 6472–6476.
- 38K. Gademann, A. Häne, M. Rueping, B. Jaun, D. Seebach, Angew. Chem. Int. Ed. 2003, 42, 1534–1537; Angew. Chem. 2003, 115, 1573–1575.
- 39B. Zagrovic, G. Jayachandran, I. S. Millett, S. Doniach, V. S. Pande, J. Mol. Biol. 2005, 353, 232–241.
- 40B. Zagrovic, W. F. van Gunsteren, Proteins Struct. Funct. Bioinf. 2006, 63, 210–218.
- 41W. F. van Gunsteren, R. Bürgi, C. Peter, X. Daura, Angew. Chem. Int. Ed. 2001, 40, 351–355; Angew. Chem. 2001, 113, 363–367.
- 42W. F. van Gunsteren, D. Bakowies, R. Baron, I. Chandrasekhar, M. Christen, X. Daura, P. Gee, D. P. Geerke, A. Glättli, P. H. Hünenberger, M. A. Kastenholz, C. Oostenbrink, M. Schenk, D. Trzesniak, N. F. A. van der Vegt, H. B. Yu, Angew. Chem. Int. Ed. 2006, 45, 4064–4092; Angew. Chem. 2006, 118, 4168–4198.
- 43J. R. Allison, W. F. van Gunsteren, ChemPhysChem 2009, 10, 3213–3228.
- 44J. Dolenc, J. H. Missimer, M. O. Steinmetz, W. F. van Gunsteren, J. Biomol. NMR 2010, 47, 221–235.
- 45B. Vögeli, J. Ying, A. Grishaev, A. Bax, J. Am. Chem. Soc. 2007, 129, 9377–9385.
- 46A. Pardi, M. Billeter, K. Wüthrich, J. Mol. Biol. 1984, 180, 741–751.
- 47R. Brüschweiler, D. A. Case, J. Am. Chem. Soc. 1994, 116, 11199–11200.
- 48A. C. Wang, A. Bax, J. Am. Chem. Soc. 1996, 118, 2483–2494.
- 49J. M. Schmidt, M. Blümel, F. Löhr, H. Rüterjans, J. Biomol. NMR 1999, 14, 1–12.
- 50A. DeMarco, M. Linàs, K. Wüthrich, Biopolymers 1978, 17, 617–636.
- 51A. DeMarco, M. Linàs, K. Wüthrich, Biopolymers 1978, 17, 2727–2742.
- 52R. J. Abraham, K. A. McLauchlan, Mol. Phys. 1962, 5, 513–523.
- 53C. M. Deber, E. R. Blout, D. A. Torchia, J. Am. Chem. Soc. 1971, 93, 4893–4897.
- 54M. T. Cung, M. Marraud, J. Nèel in 10th Prague IUPAC Micro-Symposium on Macromolecules, Prague, 1972, p. C-3.
- 55A. J. Fischman, D. H. Live, H. R. Wyssbrod, W. C. Agosta, D. Cowburn, J. Am. Chem. Soc. 1980, 102, 2533–2539.
- 56C. Pérez, F. Löhr, H. Rüterjans, J. M. Schmidt, J. Am. Chem. Soc. 2001, 123, 7081–7093.
- 57X. Daura, I. Antes, W. F. van Gunsteren, W. Thiel, A. E. Mark, Proteins Struct. Funct. Genet. 1999, 36, 542–555.
10.1002/(SICI)1097-0134(19990901)36:4<542::AID-PROT17>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 58T. S. Harvey, W. F. van Gunsteren in Techniques in Protein Chemistry IV, Academic Press, 1993, pp. 615–622.
10.1016/B978-0-12-058757-5.50072-X Google Scholar
- 59D. A. Case, Curr. Opin. Struct. Biol. 2013, 23, 172–176.
- 60W. F. van Gunsteren, R. Boelens, R. Kaptein, R. M. Scheek, E. R. P. Zuiderweg in Molecular Dynamics and Protein Structure (Ed.: ), Polycrystal Book Service, Western Springs, 1985, pp. 92–99.
- 61J. de Vlieg, R. Boelens, R. M. Scheek, R. Kaptein, W. F. van Gunsteren, Isr. J. Chem. 1986, 27, 181–188.
- 62A. E. Torda, R. M. Brunne, T. Huber, H. Kessler, W. F. van Gunsteren, J. Biomol. NMR 1993, 3, 55–66.
- 63K. Wüthrich, M. Billeter, W. Braun, J. Mol. Biol. 1983, 169, 949–961.
- 64W. F. van Gunsteren, S. R. Billeter, A. A. Eising, P. H. Hünenberger, P. Krüger, A. E. Mark, W. R. P. Scott, I. G. Tironi, Biomolecular Simulation: The GROMOS96 Manual and User Guide, Vdf Hochschulverlag AG an der ETH Zürich, Zürich, Switzerland, 1996.
- 65C. M. Fletcher, D. N. M. Jones, R. Diamond, J. Biomol. NMR 1996, 8, 292–310.
- 66M. Nilges, Proteins 1993, 17, 297–309.
- 67M. Nilges, M. J. Macias, S. I. O'Donoghue, H. Oschkinat, J. Mol. Biol. 1997, 269, 408–422.
- 68W. F. van Gunsteren in Studies in Physical and Theoretical Chemistry, Vol. 71 of Modelling of Molecular Structures and Properties (Ed.: ), Elsevier, Amsterdam, 1990, pp. 463–478.
- 69A. D. Kline, W. Braun, K. Wüthrich, J. Mol. Biol. 1988, 204, 675–724.
- 70G. M. Clore, A. T. Brünger, M. Karplus, A. M. Gronenborn, J. Mol. Biol. 1986, 191, 523–551.
- 71G. M. Clore, D. K. Sukumaran, M. Nilges, J. Zarbock, A. M. Gronenborn, EMBO J. 1987, 6, 529–537.
- 72G. M. Clore, A. M. Gronenborn, M. Nilges, D. K. Sukumaran, J. Zarbock, EMBO J. 1987, 6, 1833–1842.
- 73G. M. Clore, A. M. Gronenborn, M. Nilges, C. A. Ryan, EMBO J. 1987, 26, 8012–8023.
- 74G. M. Clore, D. K. Sukumaran, M. Nilges, A. M. Gronenborn, Biochemistry 1987, 26, 1732–1745.
- 75G. M. Clore, M. Nilges, D. K. Sukumaran, A. T. Brünger, M. Karplus, A. M. Gronenborn, EMBO J. 1986, 5, 2729–2735.
- 76W. F. van Gunsteren, M. Karplus, Macromolecules 1982, 15, 1528–1544.
- 77J. Hermans, H. J. C. Berendsen, W. F. van Gunsteren, J. P. M. Postma, Biopolymers 1984, 23, 1513–1518.
- 78B. R. Brooks, R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, M. Karplus, J. Comput. Chem. 1983, 4, 187–217.
- 79A. M. Davis, S. A. St-Gallay, G. J. Kleywegt, Drug Discovery Today 2008, 13, 831–841.
- 80J. Agirre, G. Davis, K. Wilson, K. Cowtan, Nat. Chem. Biol. 2015, 11, 303.
- 81W. G. Touw, R. P. Joosten, G. Vriend, J. Mol. Biol. 2016, 428, 1375–1393.
- 82C. A. Schiffer, R. Huber, K. Wüthrich, W. F. van Gunsteren, J. Mol. Biol. 1994, 241, 588–599.
- 83A. E. Torda, R. M. Scheek, W. F. van Gunsteren, Chem. Phys. Lett. 1989, 157, 289–294.
- 84P. Gros, W. F. van Gunsteren, W. G. J. Hol, Science 1990, 249, 1149–1152.
- 85P. Gros, W. F. van Gunsteren, Mol. Simul. 1993, 10, 377–395.
- 86C. A. Schiffer, P. Gros, W. F. van Gunsteren, Acta Crystallogr. Sect. D 1995, 51, 85–92.
- 87C. A. Schiffer, W. F. van Gunsteren, Proteins Struct. Funct. Bioinf. 1999, 36, 501–511.
10.1002/(SICI)1097-0134(19990901)36:4<501::AID-PROT14>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 88W. R. P. Scott, A. E. Mark, W. F. van Gunsteren, J. Biomol. NMR 1998, 12, 501–508.
- 89N. Hansen, F. Heller, N. Schmid, W. F. van Gunsteren, J. Biomol. NMR 2014, 60, 169–187.
- 90D. A. Pearlman, P. A. Kollman, J. Mol. Biol. 1991, 220, 457–479.
- 91U. Schmitz, A. Kumar, T. L. James, J. Am. Chem. Soc. 1992, 114, 10654–10656.
- 92U. Schmitz, B. Ulyanov, A. Kumar, T. L. James, J. Mol. Biol. 1993, 234, 373–389.
- 93D. A. Pearlman, J. Biomol. NMR 1994, 4, 1–16.
- 94D. A. Pearlman, J. Biomol. NMR 1994, 4, 279–299.
- 95A. P. Nanzer, W. F. van Gunsteren, A. E. Torda, J. Biomol. NMR 1995, 6, 313–320.
- 96A. P. Nanzer, A. E. Torda, C. Bisang, C. Weber, J. A. Robinson, W. F. van Gunsteren, J. Mol. Biol. 1997, 267, 1012–1025.
- 97A. P. E. Kunz, J. R. Allison, D. P. Geerke, B. A. C. Horta, P. H. Hünenberger, S. Riniker, N. Schmid, W. F. van Gunsteren, J. Comput. Chem. 2012, 33, 340–353.
- 98R. M. Scheek, A. E. Torda, J. Kemmink, W. F. van Gunsteren in Computational Aspects of the Study of Biological Macromolecules by Nuclear Magnetic Resonance Spectroscopy (Eds.: ), Plenum, New York, 1991, pp. 209–217.
- 99J. Fennen, A. E. Torda, W. F. van Gunsteren, J. Biomol. NMR 1995, 6, 163–170.
- 100T. Huber, W. F. van Gunsteren, J. Phys. Chem. A 1998, 102, 5937–5943.
- 101B. Hess, R. M. Scheek, J. Magn. Reson. 2003, 164, 19–27.
- 102J. W. Pitera, J. D. Chodera, J. Chem. Theory Comput. 2012, 8, 3445–3451.
- 103B. Roux, J. Weare, J. Chem. Phys. 2013, 138, 084107.
- 104A. Cavalli, C. Camilloni, M. Vendruscolo, J. Chem. Phys. 2013, 138, 094112.
- 105S. Olsson, J. Frellsen, W. Boomsma, K. V. Mardia, T. Hamelryck, PLoS One 2013, 8, e 79439.
- 106Y. Sugita, Y. Okamoto, Chem. Phys. Lett. 1999, 314, 141–151.
- 107Y. Sugita, A. Kitao, Y. Okamoto, J. Chem. Phys. 2000, 113, 6042–6051.
- 108H. Fukunishi, O. Watanabe, S. Takada, J. Chem. Phys. 2002, 116, 9058–9067.
- 109N. Schmid, J. R. Allison, J. Dolenc, A. P. Eichenberger, A.-P. E. Kunz, W. F. van Gunsteren, J. Biomol. NMR 2011, 51, 265–281.
- 110W. Rieping, M. Habeck, M. Nilges, Science 2005, 309, 303–306.
- 111X. Daura, K. Gademann, B. Jaun, D. Seebach, W. F. van Gunsteren, A. E. Mark, Angew. Chem. Int. Ed. 1999, 38, 236–240;
10.1002/(SICI)1521-3773(19990115)38:1/2<236::AID-ANIE236>3.0.CO;2-M CAS Web of Science® Google ScholarAngew. Chem. 1999, 111, 249–253.
- 112A. E. Torda, R. M. Scheek, W. F. van Gunsteren, J. Mol. Biol. 1990, 214, 223–235.
- 113C. A. Schiffer, R. Huber, K. Wüthrich, W. F. van Gunsteren, J. Mol. Biol. 1994, 241, 588–599.
- 114D. Seebach, P. E. Ciceri, M. Overhand, B. Jaun, D. Rigo, L. Oberer, U. Hommel, R. Amstutz, H. Widmer, Helv. Chim. Acta 1996, 79, 2043–2066.
- 115D. Seebach, S. Abele, K. Gademann, G. Guichard, T. Hintermann, B. Jaun, J. L. Matthews, J. V. Schreiber, L. Oberer, U. Hommel, H. Widmer, Helv. Chim. Acta 1998, 81, 932–982.
- 116D. Seebach, K. Gademann, J. V. Schreiber, J. L. Matthews, T. Hintermann, B. Jaun, L. Oberer, U. Hommel, H. Widmer, Helv. Chim. Acta 1997, 80, 2033–2038.
- 117T. Huber, A. E. Torda, W. F. van Gunsteren, J. Comput.-Aided Mol. Des. 1994, 8, 695–708.
- 118A. Laio, M. Parrinello, Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566.
- 119M. Christen, B. Keller, W. F. van Gunsteren, J. Biomol. NMR 2007, 39, 265–273.
- 120Z. Gattin, J. Zaugg, W. F. van Gunsteren, ChemPhysChem 2010, 11, 830–835.
- 121J. H. Missimer, J. Dolenc, M. O. Steinmetz, W. F. van Gunsteren, Protein Sci. 2010, 19, 2462–2474.
- 122Z. Lin, W. F. van Gunsteren, J. Chem. Phys. 2015, 143, 034110.
- 123A. Kuzmanic, N. S. Pannu, B. Zagrovic, Nat. Commun. 2014, 5, 3220.
- 124Z. Dauter, A. Wlodawer, W. Minor, M. Jaskolski, B. Rupp, IUCrJ 2014, 1, 179–193.
- 125H. van den Bedem, J. S. Fraser, Nat. Methods 2015, 12, 307–318.
- 126M. O. Steinmetz, I. Jelesarov, W. M. Matousek, S. Honnappa, W. Jahnke, J. H. Missimer, S. Frank, A. T. Alexandrescu, R. A. Kammerer, Proc. Natl. Acad. Sci. USA 2007, 104, 7062–7067.
- 127C. Oostenbrink, A. Villa, A. E. Mark, W. F. van Gunsteren, J. Comput. Chem. 2004, 25, 1656–1676.
- 128M. H. Lerche, F. M. Poulsen, Protein Sci. 1998, 7, 2490–2498.
- 129L. J. Smith, W. F. van Gunsteren, J. R. Allison, Protein Sci. 2013, 22, 56–64.
- 130L. F. Pacios, C. Gómez-Casado, L. Tordesillas, A. Palacin, R. Sánchez-Monge, A. Díaz-Perales, J. Comput. Chem. 2012, 33, 1831–1844.
- 131L. J. Smith, Y. Roby, J. R. Allison, W. F. van Gunsteren, Biochemistry 2013, 52, 5024–5038.
- 132G. W. Han, J. Y. Lee, H. K. Song, C. S. Chang, K. Min, J. Moon, D. H. Shin, M. L. Kopka, M. R. Sawaya, H. S. Yuan, T. D. Kim, J. Choe, D. Lim, H. J. Moon, S. W. Suh, J. Mol. Biol. 2001, 308, 263–278.
- 133D. Trzesniak, W. F. van Gunsteren, Protein Sci. 2006, 15, 2544–2551.
- 134B. T. Burnley, P. V. Afonine, P. D. Adams, P. Gros, eLife 2012, 1, e 00311.
- 135A. Wlodawer, W. Minor, Z. Dauter, M. Jaskolski, FEBS J. 2008, 275, 1–21.
- 136A. Wlodawer, W. Minor, Z. Dauter, M. Jaskolski, FEBS J. 2013, 280, 5705–5736.
- 137R. P. Joosten, K. Joosten, G. N. Murshudov, A. Perrakis, Acta Crystallogr. Sect. D 2012, 68, 484–496.
- 138G. T. Montelione, M. Nilges, A. Bax, P. Güntert, T. Herrmann, J. S. Richardson, C. D. Schwieters, W. F. Vranken, G. W. Vuister, D. S. Wishart, H. M. Berman, G. J. Kleywegt, J. L. Markley, Structure 2013, 21, 1563–1570.
- 139P. D. Adams, K. Aertgeerts, C. Bauer, J. A. Bell, H. M. Berman, T. N. Bhat, J. M. Blaney, E. Bolton, G. Bricogne, D. Brown, S. K. Burley, D. A. Case, K. L. Clark, T. Darden, P. Emsley, V. A. Feher, Z. Feng, C. R. Groom, S. F. Harris, J. Hendle, T. Holder, A. Joachimiak, G. J. Kleywegt, T. Krojer, J. Marcotrigiano, A. E. Mark, J. L. Markley, M. Miller, W. Minor, G. T. Montelione, G. Murshudov, A. Nakagawa, H. Nakamura, A. Nicholls, M. Nicklaus, R. T. Nolte, A. K. Padyana, C. E. Peishoff, S. Pieniazek, R. J. Read, C. Shao, S. Sheriff, O. Smart, S. Soisson, J. Spurlino, T. Stouch, R. Svobodova, W. Tempel, T. C. Terwilliger, D. Tronrud, S. Velankar, S. C. Ward, G. L. Warren, J. D. Westbrook, P. Williams, H. Yang, J. Young, Structure 2016, 24, 502–508.
- 140G. Miller, Science 2006, 314, 1856–1857.