Mild C−H/C−C Activation by Z-Selective Cobalt Catalysis
Daniel Zell
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
These authors contributed equally to this work.
Search for more papers by this authorQingqing Bu
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Milica Feldt
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Lutz Ackermann
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorDaniel Zell
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
These authors contributed equally to this work.
Search for more papers by this authorQingqing Bu
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Milica Feldt
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Lutz Ackermann
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorGraphical Abstract
Abstract
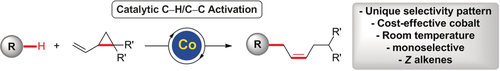
Cationic cobalt complexes enable unprecedented cobalt-catalyzed C−H/C−C functionalizations with unique selectivity features. The versatile cobalt catalyst proved broadly applicable, enabled efficient C−H/C−C cleavage at room temperature, and delivered Z-alkenes with excellent diastereocontrol.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201601778-sup-0001-misc_information.pdf8.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Representative recent reviews on C−H activation:
- 1aJ. K. Kim, K. Shin, S. Chang, Top. Organomet. Chem. 2016, 55, 29–51;
- 1bC. Borie, L. Ackermann, M. Nechab, Chem. Soc. Rev. 2016, 45, 1368–1386;
- 1cO. Daugulis, J. Roane, L. D. Tran, Acc. Chem. Res. 2015, 48, 1053–1064;
- 1dJ. Li, L. Ackermann, Nat. Chem. 2015, 7, 686–687;
- 1eK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040–1052;
- 1fY. Segawa, T. Maekawa, K. Itami, Angew. Chem. Int. Ed. 2015, 54, 66–81; Angew. Chem. 2015, 127, 68–83;
- 1gG. Song, X. Li, Acc. Chem. Res. 2015, 48, 1007–1020;
- 1hN. Kuhl, N. Schroeder, F. Glorius, Adv. Synth. Catal. 2014, 356, 1443–1460;
- 1iS. A. Girard, T. Knauber, C.-J. Li, Angew. Chem. Int. Ed. 2014, 53, 74–100; Angew. Chem. 2014, 126, 76–103;
- 1jJ. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369–375;
- 1kT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212–11222;
- 1lR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242–3272;
- 1mL. Ackermann, R. Vicente, A. Kapdi, Angew. Chem. Int. Ed. 2009, 48, 9792–9826; Angew. Chem. 2009, 121, 9976–10011;
- 1nR. G. Bergman, Nature 2007, 446, 391–393, and references therein.
- 2J. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960–9009; Angew. Chem. 2012, 124, 9092–9142.
- 3L. Ackermann, Org. Process Res. Dev. 2015, 19, 260–269.
- 4L. G. Mercier, M. Leclerc, Acc. Chem. Res. 2013, 46, 1597–1605.
- 5Recent reviews:
- 5aL. Souillart, N. Cramer, Chem. Rev. 2015, 115, 9410–9464;
- 5bT. Seiser, T. Saget, D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 7740–7752; Angew. Chem. 2011, 123, 7884–7896;
- 5cM. Murakami, T. Matsuda, Chem. Commun. 2011, 47, 1100–1105;
- 5dT. Xu, A. Dermenci, G. Dong, Top. Curr. Chem. 2014, 346, 233–257;
- 5ea recent elegant example of cobalt-catalyzed C−C functionalization: E. Ozkal, B. Cacherat, B. Morandi, ACS Catal. 2015, 5, 6458–6462.
- 6Examples of C−C activation by means of rhodium catalysis:
- 6aT. Matsuda, K. Kato, T. Goya, S. Shimada, M. Murakami, Chem. Eur. J. 2016, 22, 1941–1943;
- 6bL. Deng, T. Xu, H. Li, G. Dong, J. Am. Chem. Soc. 2016, 138, 369–374;
- 6cX. Zhou, G. Dong, J. Am. Chem. Soc. 2015, 137, 13715–13721;
- 6dR. Zeng, G. Dong, J. Am. Chem. Soc. 2015, 137, 1408–1411;
- 6eA. Yada, S. Fujita, M. Murakami, J. Am. Chem. Soc. 2014, 136, 7217–7220;
- 6fH. M. Ko, G. Dong, Nat. Chem. 2014, 6, 739–744;
- 6gN. Ishida, W. Ikemoto, M. Murakami, J. Am. Chem. Soc. 2014, 136, 5912–5915;
- 6hL. Souillart, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 9640–9644; Angew. Chem. 2014, 126, 9794–9798;
- 6iL. Ding, N. Ishida, M. Murakami, K. Morokuma, J. Am. Chem. Soc. 2014, 136, 169–178;
- 6jL. Souillart, E. Parker, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 3001–3005; Angew. Chem. 2014, 126, 3045–3049;
- 6kL. Souillart, N. Cramer, Chem. Sci. 2014, 5, 837–840;
- 6lY. Masuda, M. Hasegawa, M. Yamashita, K. Nozaki, N. Ishida, M. Murakami, J. Am. Chem. Soc. 2013, 135, 7142–7145;
- 6mN. Ishida, S. Sawano, Y. Masuda, M. Murakami, J. Am. Chem. Soc. 2012, 134, 17502–17504;
- 6nT. Seiser, N. Cramer, J. Am. Chem. Soc. 2010, 132, 5340–5341;
- 6oL. Liu, N. Ishida, M. Murakami, Angew. Chem. Int. Ed. 2012, 51, 2485–2488; Angew. Chem. 2012, 124, 2535–2538;
- 6pM. Murakami, H. Amii, Y. Ito, Nature 1994, 370, 540–541, and references therein.
- 7A. Masarwa, D. Didier, T. Zabrodski, M. Schinkel, L. Ackermann, I. Marek, Nature 2014, 505, 199–203.
- 8
- 8aJ.-Q. Wu, Z.-P. Qiu, S.-S. Zhang, J.-G. Liu, Y.-X. Lao, L.-Q. Gu, Z.-S. Huang, J. Li, H. Wang, Chem. Commun. 2015, 51, 77–80;
- 8bA. Masarwa, M. Weber, R. Sarpong, J. Am. Chem. Soc. 2015, 137, 6327–6334;
- 8cT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 6320–6323; Angew. Chem. 2009, 121, 6438–6441;
- 8dC. Aïssa, A. Fürstner, J. Am. Chem. Soc. 2007, 129, 14836–14837.
- 9Recent authoritative reviews on the use of inexpensive first-row transition metal catalysts for C−H bond functionalization:
- 9aE. Nakamura, T. Hatakeyama, S. Ito, K. Ishizuka, L. Ilies, M. Nakamura, Org. React. 2014, 83, 1–209;
- 9bL. Ackermann, J. Org. Chem. 2014, 79, 8948–8954;
- 9cK. Gao, N. Yoshikai, Acc. Chem. Res. 2014, 47, 1208–1219;
- 9dN. Yoshikai, Synlett 2011, 1047–1051;
- 9eY. Nakao, Chem. Rec. 2011, 11, 242–251;
- 9fE. Nakamura, N. Yoshikai, J. Org. Chem. 2010, 75, 6061–6067;
- 9gA. Kulkarni, O. Daugulis, Synthesis 2009, 4087–4109, and references therein.
- 10M. Moselage, J. Li, L. Ackermann, ACS Catal. 2016, 6, 498–525.
- 11
- 11aB. Sun, T. Yoshino, M. Kanai, S. Matsunaga, Angew. Chem. Int. Ed. 2015, 54, 12968–12972; Angew. Chem. 2015, 127, 13160–13164;
- 11bY. Suzuki, B. Sun, T. Yoshino, M. Kanai, S. Matsunaga, Tetrahedron 2015, 71, 4552–4556;
- 11cY. Suzuki, B. Sun, K. Sakata, T. Yoshino, S. Matsunaga, M. Kanai, Angew. Chem. Int. Ed. 2015, 54, 9944–9947; Angew. Chem. 2015, 127, 10082–10085;
- 11dB. Sun, T. Yoshino, S. Matsunaga, M. Kanai, Chem. Commun. 2015, 51, 4659–4661;
- 11eH. Ikemoto, T. Yoshino, K. Sakata, S. Matsunaga, M. Kanai, J. Am. Chem. Soc. 2014, 136, 5424–5431;
- 11fB. Sun, T. Yoshino, S. Matsunaga, M. Kanai, Adv. Synth. Catal. 2014, 356, 1491–1495;
- 11gT. Andou, Y. Saga, H. Komai, S. Matsunaga, M. Kanai, Angew. Chem. Int. Ed. 2013, 52, 3213–3216; Angew. Chem. 2013, 125, 3295–3298;
- 11hT. Yoshino, H. Ikemoto, S. Matsunaga, M. Kanai, Angew. Chem. Int. Ed. 2013, 52, 2207–2211; Angew. Chem. 2013, 125, 2263–2267.
- 12
- 12aJ. R. Hummel, J. A. Ellman, Org. Lett. 2015, 17, 2400–2403;
- 12bJ. R. Hummel, J. A. Ellman, J. Am. Chem. Soc. 2015, 137, 490–498.
- 13
- 13aA. B. Pawar, S. Chang, Org. Lett. 2015, 17, 660–663;
- 13bP. Patel, S. Chang, ACS Catal. 2015, 5, 853–858;
- 13cJ. Park, S. Chang, Angew. Chem. Int. Ed. 2015, 54, 14103–14107; Angew. Chem. 2015, 127, 14309–14313.
- 14
- 14aA. Lerchen, S. Vásquez-Céspedes, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 3208–3211; Angew. Chem. 2016, 128, 3261–3265;
- 14bD. Zhao, J. H. Kim, L. Stegemann, C. A. Strassert, F. Glorius, Angew. Chem. Int. Ed. 2015, 54, 4508–4511; Angew. Chem. 2015, 127, 4591–4594;
- 14cT. Gensch, S. Vasquez-Cespedes, D.-G. Yu, F. Glorius, Org. Lett. 2015, 17, 3714–3717;
- 14dD.-G. Yu, T. Gensch, F. D. Azambuja, S. Vásquez-Céspedes, F. Glorius, J. Am. Chem. Soc. 2014, 136, 17722–17725.
- 15Z.-Z. Zhang, B. Liu, C.-Y. Wang, B.-F. Shi, Org. Lett. 2015, 17, 4094–4097.
- 16
- 16aY. Liang, N. Jiao, Angew. Chem. Int. Ed. 2016, 55, 4035–4039; Angew. Chem. 2016, 128, 4103–4107;
- 16bY. Liang, Y.-F. Liang, C. Tang, Y. Yuan, N. Jiao, Chem. Eur. J. 2015, 21, 16395–16399.
- 17
- 17aR. Mei, H. Wang, S. Warratz, S. A. Macgregor, L. Ackermann, Chem. Eur. J. 2016, 22, DOI: 10.1002/chem.201601101;
- 17bR. Mei, J. Loup, L. Ackermann, ACS Catal. 2016, 6, 793–797;
- 17cJ. Li, L. Ackermann, Angew. Chem. Int. Ed. 2015, 54, 8551–8554; Angew. Chem. 2015, 127, 8671–8674;
- 17dM. Moselage, N. Sauermann, J. Koeller, W. Liu, D. Gelman, L. Ackermann, Synlett 2015, 1596–1600;
- 17eN. Sauermann, M. J. González, L. Ackermann, Org. Lett. 2015, 17, 5316–5319;
- 17fW. Ma, L. Ackermann, ACS Catal. 2015, 5, 2822–2825;
- 17gJ. Li, L. Ackermann, Angew. Chem. Int. Ed. 2015, 54, 3635–3638; Angew. Chem. 2015, 127, 3706–3709.
- 18
- 18aS. Prakash, K. Muralirajan, C.-H. Cheng, Angew. Chem. Int. Ed. 2016, 55, 1844–1848; Angew. Chem. 2016, 128, 1876–1880;
- 18bT. T. Nguyen, L. Grigorjeva, O. Daugulis, ACS Catal. 2016, 6, 551–554;
- 18cN. Barsu, M. Sen, J. R. Premkumar, B. Sundararaju, Chem. Commun. 2016, 52, 1338–1341;
- 18dL.-B. Zhang, X.-Q. Hao, Z.-J. Liu, X.-X. Zheng, S.-K. Zhang, J.-L. Niu, M.-P. Song, Angew. Chem. Int. Ed. 2015, 54, 10012–10015; Angew. Chem. 2015, 127, 10150–10153;
- 18eL.-B. Zhang, X.-Q. Hao, S.-K. Zhang, Z.-J. Liu, X.-X. Zheng, J.-F. Gong, J.-L. Niu, M.-P. Song, Angew. Chem. Int. Ed. 2015, 54, 272–275; Angew. Chem. 2015, 127, 274–277;
- 18fX. Wu, K. Yang, Y. Zhao, H. Sun, G. Li, H. Ge, Nat. Commun. 2015, 6, 6462;
- 18gL. Grigorjeva, O. Daugulis, Org. Lett. 2015, 17, 1204–1207;
- 18hL. Grigorjeva, O. Daugulis, Angew. Chem. Int. Ed. 2014, 53, 10209–10212; Angew. Chem. 2014, 126, 10373–10376, and references therein.
- 19
- 19aM. Moselage, N. Sauermann, S. C. Richter, L. Ackermann, Angew. Chem. Int. Ed. 2015, 54, 6352–6355; Angew. Chem. 2015, 127, 6450–6453;
- 19bW. Song, L. Ackermann, Angew. Chem. Int. Ed. 2012, 51, 8251–8254; Angew. Chem. 2012, 124, 8376–8379.
- 20For detailed information, see the Supporting Information.
- 21
- 21aL. Ackermann, Acc. Chem. Res. 2014, 47, 281–295;
- 21bL. Ackermann, Chem. Rev. 2011, 111, 1315–1345.
- 22A. D. Becke, J. Chem. Phys. 1993, 98, 5648.
- 23P. Stephens, F. J. Devlin, C. Chabalowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11623–11627.
- 24S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465.
- 25S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 26S. Kozuch, S. Shaik, Acc. Chem. Res. 2011, 44, 101–110.
- 27Vinylcyclopropanes 2 lacking electron-withdrawing groups gave only unsatisfactory yields.
- 28J. Wencel-Delord, T. Droege, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740–4761.