Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction
Graphical Abstract
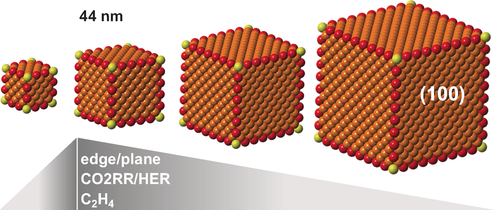
On the edge: Cu nanocrystal cubes and spheres with different sizes were synthesized by means of colloidal chemistry. The highest selectivity towards the CO2 reduction reaction (CO2RR) and ethylene was found in Cu cubes with 44 nm edge length. The size-dependent trend of the catalytic activity suggests the key role played by edge sites in CO2RR.
Abstract
Favoring the CO2 reduction reaction (CO2RR) over the hydrogen evolution reaction and controlling the selectivity towards multicarbon products are currently major scientific challenges in sustainable energy research. It is known that the morphology of the catalyst can modulate catalytic activity and selectivity, yet this remains a relatively underexplored area in electrochemical CO2 reduction. Here, we exploit the material tunability afforded by colloidal chemistry to establish unambiguous structure/property relations between Cu nanocrystals and their behavior as electrocatalysts for CO2 reduction. Our study reveals a non-monotonic size-dependence of the selectivity in cube-shaped copper nanocrystals. Among 24 nm, 44 nm and 63 nm cubes tested, the cubes with 44 nm edge length exhibited the highest selectivity towards CO2RR (80 %) and faradaic efficiency for ethylene (41 %). Statistical analysis of the surface atom density suggests the key role played by edge sites in CO2RR.