Dibenzo[a,j]phenazine-Cored Donor–Acceptor–Donor Compounds as Green-to-Red/NIR Thermally Activated Delayed Fluorescence Organic Light Emitters
Corresponding Author
Dr. Przemyslaw Data
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Faculty of Chemistry, Silesian University of Technology, M. Strzody 9, 44-100 Poland
Search for more papers by this authorPiotr Pander
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Faculty of Chemistry, Silesian University of Technology, M. Strzody 9, 44-100 Poland
Search for more papers by this authorMasato Okazaki
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Youhei Takeda
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorProf. Dr. Satoshi Minakata
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorProf. Dr. Andrew P. Monkman
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Search for more papers by this authorCorresponding Author
Dr. Przemyslaw Data
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Faculty of Chemistry, Silesian University of Technology, M. Strzody 9, 44-100 Poland
Search for more papers by this authorPiotr Pander
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Faculty of Chemistry, Silesian University of Technology, M. Strzody 9, 44-100 Poland
Search for more papers by this authorMasato Okazaki
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Youhei Takeda
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorProf. Dr. Satoshi Minakata
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka, 565-0871 Japan
Search for more papers by this authorProf. Dr. Andrew P. Monkman
Physics Department, Durham University, South Road, Durham, DH1 3LE UK
Search for more papers by this authorGraphical Abstract
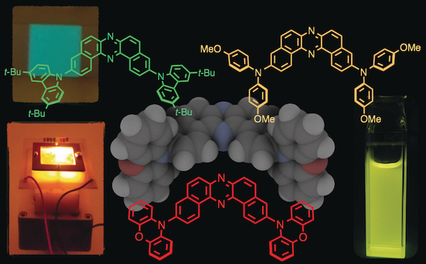
Photophysics: A series of U-shaped donor–acceptor–donor emissive compounds based on the electron-accepting unit dibenzo[a,j]phenazine has been developed. Static and dynamic photophysical investigations of these compounds revealed their detailed thermally activated delayed fluorescence properties. The external quantum efficiency of the organic light-emitting diodes fabricated with the new materials reached values up to 16 %.
Abstract
A new family of thermally activated delayed fluorescence (TADF) emitters based on U-shaped D-A-D architecture with a novel accepting unit has been developed. All investigated compounds have small singlet-triplet energy splitting (ΔEST) ranging from 0.02 to 0.20 eV and showed efficient TADF properties. The lowest triplet state of the acceptor unit plays the key role in the TADF mechanism. OLEDs fabricated with these TADF emitters achieved excellent efficiencies up to 16 % external quantum efficiency (EQE).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600113-sup-0001-misc_information.pdf18.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. W. Tang, S. A. Van Slyke, Appl. Phys. Lett. 1987, 51, 913–915.
- 2
- 2aS. R. Forrest, Nature 2004, 428, 911–918;
- 2b Organic Light Emitting Devices, Synthesis Properties and Applications, (Eds.: ), Wiley-VCH, Weinheim, 2006;
- 2c OLED Fundamentals, Materials, Devices and Processing of Organic Light-Emitting Diodes, (Eds.: ), CRC Press, Boca Raton, FL, 2015.
- 3 Highly Efficient OLEDs with Phosphorescent Materials, (Ed.: ), Wiley-VCH, Weinheim, 2008.
- 4
- 4aA. R. Brown, K. Pichler, N. C. Greenham, D. D. C. Bradley, R. H. Friend, A. B. Holmes, Chem. Phys. Lett. 1993, 210, 61–66;
- 4bM. A. Baldo, D. F. O'Brien, M. E. Thompson, S. R. Forrest, Phys. Rev. B 1999, 60, 14422–14428.
- 5J. Partee, E. L. Frankevich, B. Uhlhorn, J. Shinar, Y. Ding, T. J. Barton, Phys. Rev. Lett. 1999, 82, 3673–3676.
- 6
- 6aS. Sinha, A. P. Monkman, Appl. Phys. Lett. 2003, 82, 4651–4653;
- 6bC.-J. Chiang, A. Kimyonok, M. K. Etherington, G. C. Griffiths, V. Jankus, F. Turksoy, A. P. Monkman, Adv. Funct. Mater. 2013, 23, 739–746.
- 7C. A. Parker, C. G. Hatchard, Trans. Faraday Soc. 1961, 57, 1894.
- 8H. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature 2012, 492, 234–238.
- 9
- 9aF. B. Dias, K. N. Bourdakos, V. Jankus, K. C. Moss, K. T. Kamtekar, V. Bhalla, J. Santos, M. R. Bryce, A. P. Monkman, Adv. Mater. 2013, 25, 3707–3714;
- 9bV. Jankus, P. Data, D. Graves, C. McGuinness, J. Santos, M. R. Bryce, F. B. Dias, A. P. Monkman, Adv. Funct. Mater. 2014, 24, 6178–6186.
- 10A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato, C. Adachi, Adv. Mater. 2009, 21, 4802–4806.
- 11
- 11aA. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki, C. Adachi, Appl. Phys. Lett. 2011, 98, 083302;
- 11bG. Méhes, H. Nomura, Q. Zhang, T. Nakagawa, C. Adachi, Angew. Chem. Int. Ed. 2012, 51, 11311–11315; Angew. Chem. 2012, 124, 11473–11477;
- 11cQ. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki, C. Adachi, J. Am. Chem. Soc. 2012, 134, 14706–14709;
- 11dJ. Li, T. Nakagawa, J. MacDonald, Q. Zhang, H. Nomura, H. Miyazaki, C. Adachi, Adv. Mater. 2013, 25, 3319–3323;
- 11eJ. Lee, K. Shizu, H. Tanaka, H. Nomura, T. Yasuda, C. Adachi, J. Mater. Chem. C 2013, 1, 4599–4604;
- 11fS. Y. Lee, T. Yasuda, Y. S. Yang, Q. Zhang, C. Adachi, Angew. Chem. Int. Ed. 2014, 53, 6402–6406; Angew. Chem. 2014, 126, 6520–6524;
- 11gT. Takahashi, K. Shizu, T. Yasuda, K. Togashi, C. Adachi, Sci. Technol. Adv. Mater. 2014, 15, 034202;
- 11hS. Y. Lee, T. Yasuda, I. S. Park, C. Adachi, Dalton Trans. 2015, 44, 8356–8359;
- 11iH. Kaji, H. Suzuki, T. Fukushima, K. Shizu, K. Suzuki, S. Kubo, T. Komino, H. Oiwa, F. Suzuki, A. Wakamiya, Y. Murata, C. Adachi, Nat. Commun. 2015, 6, 8476;
- 11jK. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi, H. Kaji, Angew. Chem. Int. Ed. 2015, 54, 15231–15235; Angew. Chem. 2015, 127, 15446–15450.
- 12
- 12aY. J. Cho, S. K. Jeon, B. D. Chin, E. Yu, J. Y. Lee, Angew. Chem. Int. Ed. 2015, 54, 5201–5204; Angew. Chem. 2015, 127, 5290–5293;
- 12bK. Albrecht, K. Matsuoka, K. Fujita, K. Yamamoto, Angew. Chem. Int. Ed. 2015, 54, 5677–5682; Angew. Chem. 2015, 127, 5769–5774;
- 12cK. Kawasumi, T. Wu, T. Zhu, H. S. Chae, T. V. Voorhis, M. A. Baldo, T. M. Swager, J. Am. Chem. Soc. 2015, 137, 11908–11911.
- 13Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang, W. Huang, Adv. Mater. 2014, 26, 7931–7958, and references therein.
- 14Y. Takeda, M. Okazaki, S. Minakata, Chem. Commun. 2014, 50, 10291–10294.
- 15B. T. Lim, S. Okajima, A. K. Chandra, E. C. Lim, Chem. Phys. Lett. 1981, 79, 22–27.
- 16
- 16aK. Goushi, K. Yoshida, K. Sato, C. Adachi, Nat. Photonics 2012, 6, 253–258;
- 16bD. Graves, V. Jankus, F. B. Dias, A. Monkman, Adv. Funct. Mater. 2014, 24, 2343–2351.
- 17
- 17aH. Tanaka, K. Shizu, H. Nakanotani, C. Adachi, Chem. Mater. 2013, 25, 3766–3771;
- 17bJ. Li, T. Nakagawa, J. MacDonald, Q. Zhang, H. Nomura, H. Miyazaki, C. Adachi, Adv. Mater. 2013, 25, 3319–3323;
- 17cQ. Zhang, H. Kuwabara, W. J., Jr. Potscavage, S. Huang, Y. Hatae, T. Shibata, C. Adachi, J. Am. Chem. Soc. 2014, 136, 18070–18081;
- 17dS. Wang, X. Yan, Z. Cheng, H. Zhang, Y. Liu, Y. Wang, Angew. Chem. Int. Ed. 2015, 54, 13068–13072; Angew. Chem. 2015, 127, 13260–13264.
- 18H. A. Al Attar, A. P. Monkman, M. Tavasli, S. Bettington, M. R. Bryce, Appl. Phys. Lett. 2005, 86, 121101.