Cyclobutane and Cyclobutene Synthesis: Catalytic Enantioselective [2+2] Cycloadditions
Yao Xu
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorMichael L. Conner
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorCorresponding Author
Prof. M. Kevin Brown
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)Search for more papers by this authorYao Xu
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorMichael L. Conner
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorCorresponding Author
Prof. M. Kevin Brown
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)
Department of Chemistry, Indiana University, 800 E. Kirkwood Ave., Bloomington, IN 47405 (USA)Search for more papers by this authorGraphical Abstract
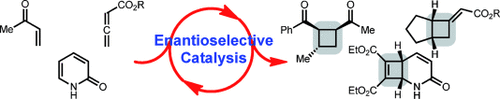
Squared away: Cyclobutanes and cyclobutenes are important structural motifs found in numerous biologically significant molecules, and they are useful intermediates for chemical synthesis. Consequently, catalytic enantioselective [2+2] cycloadditions to access cyclobutanes and cyclobutenes have emerged as an attractive target for method development. The advances made in catalytic enantioselective [2+2] cycloadditions are described herein.
Abstract
Cyclobutanes and cyclobutenes are important structural motifs found in numerous biologically significant molecules, and they are useful intermediates for chemical synthesis. Consequently, [2+2] cycloadditions to access cyclobutanes and cyclobutenes have been established to be particularly useful transformations. Within the last 10 years, an increase in the frequency of publications for catalytic enantioselective [2+2] cycloadditions has occurred. These reactions provide access to a wide array of enantiomerically enriched chemical diversity that was not previously attainable. Described in this review are the advances made in catalytic enantioselective [2+2] cycloadditions to access cyclobutanes and cyclobutenes.
References
- 1
- 1aE. M. Carreira, L. Kvaerno, Classics in Stereoselective Synthesis, Wiley-VCH, Weinheim, 2009;
- 1bK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 2002, 41, 1668–1698;
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1742–1773.
- 2For a prior review that partially covers catalytic enantioselective [2+2] cycloadditions, see: F. Secci, A. Frongia, P. Piras, Molecules 2013, 18, 15541–15572.
- 3
- 3aD. Belluš, B. Ernst, Angew. Chem. Int. Ed. Engl. 1988, 27, 797–827; Angew. Chem. 1988, 100, 820–850;
- 3bJ. C. Namyslo, D. E. Kaufmann, Chem. Rev. 2003, 103, 1485–1538;
- 3cE. Lee-Ruff, G. Mladenova, Chem. Rev. 2003, 103, 1449–1484.
- 4B. M. Trost, Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281; Angew. Chem. 1995, 107, 285–307.
- 5
- 5aA. Sergeiko, V. V. Poroikov, L. O. Hanus, V. M. Dembitsky, Open Med. Chem. J. 2008, 2, 26–37;
- 5bV. M. Dembitsky, J. Nat. Med. 2007, 62, 1–33.
- 6One analysis found >1600 cyclobutane-containing natural products. T. P. Yoon, ACS Catal. 2013, 3, 895–902.
- 7For references regarding the examples illustrated in Scheme 1 b, see:
- 7aS.-J. Piao, Y.-L. Song, W.-H. Jiao, F. Yang, X.-F. Liu, W.-S. Chen, B.-N. Han, H.-W. Lin, Org. Lett. 2013, 15, 3526–3529;
- 7bP.-S. Yang, M.-J. Cheng, C.-F. Peng, J.-J. Chen, I.-S. Chen, J. Nat. Prod. 2009, 72, 53–58;
- 7cJ. S. Sinninghe Damsté, M. Strous, W. I. C. Rijpstra, E. C. Hopmans, J. A. J. Geenevasen, A. C. T. van Duin, L. A. van Niftrik, M. S. M. Jetten, Nature 2002, 419, 708–712;
- 7dN. Aimi, M. Inaba, M. Watanabe, S. Shibata, Tetrahedron 1969, 25, 1825–1838.
- 8For references regarding the examples illustrated in Scheme 1 c, see:
- 8aD. C. Blakemore, J. S. Bryans, P. Carnell, C. L. Carr, N. E. A. Chessum, M. J. Field, N. Kinsella, S. A. Osborne, A. N. Warren, S. C. Williams, Bioorg. Med. Chem. Lett. 2010, 20, 461–464;
- 8bJ. P. Vilaine, C. Thollon, N. Villenueve, J. L. Peglion, Eur. Heart J. Suppl. 2003, 5, G 26–G35.
- 9
- 9aE. M. Carreira, T. C. Fessard, Chem. Rev. 2014, 114, 8257–8322;
- 9bC. M. Marson, Chem. Soc. Rev. 2011, 40, 5514;
- 9cK. A. Brameld, B. Kuhn, D. C. Reuter, M. Stahl, J. Chem. Inf. Model. 2008, 48, 1–24.
- 10For example, see: F. Kleinbeck, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 9178–9179.
- 11For example, see: C. M. Reeves, C. Eidamshaus, J. Kim, B. M. Stoltz, Angew. Chem. Int. Ed. 2013, 52, 6718–6721; Angew. Chem. 2013, 125, 6850–6853.
- 12
- 12aT. Bach, J. P. Hehn, Angew. Chem. Int. Ed. 2011, 50, 1000–1045; Angew. Chem. 2011, 123, 1032–1077;
- 12bJ. D. Winkler, C. M. Bowen, F. Liotta, Chem. Rev. 1995, 95, 2003–2020.
- 13R. Brimioulle, D. Lenhart, M. M. Maturi, T. Bach, Angew. Chem. Int. Ed. 2015, 54, 3872–3890; Angew. Chem. 2015, 127, 3944–3963.
- 14
- 14aM. M. Maturi, M. Wenninger, R. Alonso, A. Bauer, A. Pöthig, E. Riedle, T. Bach, Chem. Eur. J. 2013, 19, 7461–7472;
- 14bC. Müller, A. Bauer, M. M. Maturi, M. C. Cuquerella, M. A. Miranda, T. Bach, J. Am. Chem. Soc. 2011, 133, 16689–16697;
- 14cC. Müller, A. Bauer, T. Bach, Angew. Chem. Int. Ed. 2009, 48, 6640–6642; Angew. Chem. 2009, 121, 6767–6769; For selected related earlier studies with stoichiometric quantities of a chiral promoter, see:
- 14dT. Bach, H. Bergmann, B. Grosch, K. Harms, J. Am. Chem. Soc. 2002, 124, 7982–7990;
- 14eT. Bach, H. Bergmann, J. Am. Chem. Soc. 2000, 122, 11525–11526;
- 14fT. Bach, H. Bergmann, K. Harms, Angew. Chem. Int. Ed. 2000, 39, 2302–2304;
10.1002/1521-3773(20000703)39:13<2302::AID-ANIE2302>3.0.CO;2-6 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2391–2393.
- 15D. F. Cauble, V. Lynch, M. J. Krische, J. Org. Chem. 2003, 68, 15–21.
- 16M. M. Maturi, T. Bach, Angew. Chem. Int. Ed. 2014, 53, 7661–7664; Angew. Chem. 2014, 126, 7793–7796.
- 17R. Alonso, T. Bach, Angew. Chem. Int. Ed. 2014, 53, 4368–4371; Angew. Chem. 2014, 126, 4457–4460.
- 18F. D. Lewis, S. V. Barancyk, J. Am. Chem. Soc. 1989, 111, 8653–8661.
- 19
- 19aR. Brimioulle, H. Guo, T. Bach, Chem. Eur. J. 2012, 18, 7552–7560;
- 19bH. Guo, E. Herdtweck, T. Bach, Angew. Chem. Int. Ed. 2010, 49, 7782–7785; Angew. Chem. 2010, 122, 7948–7951.
- 20E. J. Corey, Angew. Chem. Int. Ed. 2009, 48, 2100–2117; Angew. Chem. 2009, 121, 2134–2151.
- 21R. Brimioulle, T. Bach, Angew. Chem. Int. Ed. 2014, 53, 12921–12924; Angew. Chem. 2014, 126, 13135–13138.
- 22R. Brimioulle, T. Bach, Science 2013, 342, 840–843.
- 23
- 23aN. Vallavoju, S. Selvakumar, S. Jockusch, M. P. Sibi, J. Sivaguru, Angew. Chem. Int. Ed. 2014, 53, 5604–5608; Angew. Chem. 2014, 126, 5710–5714;
- 23bN. Vallavoju, S. Selvakumar, S. Jockusch, M. T. Prabhakaran, M. P. Sibi, J. Sivaguru, Adv. Synth. Catal. 2014, 356, 2763–2768.
- 24J. Du, K. L. Skubi, D. M. Schultz, T. P. Yoon, Science 2014, 344, 392–396.
- 25
- 25aref. [6];
- 25bE. L. Tyson, E. P. Farney, T. P. Yoon, Org. Lett. 2012, 14, 1110–1113;
- 25cJ. Du, T. P. Yoon, J. Am. Chem. Soc. 2009, 131, 14604–14605;
- 25dM. A. Ischay, M. E. Anzovino, J. Du, T. P. Yoon, J. Am. Chem. Soc. 2008, 130, 12886–12887.
- 26For selected examples of non-enantioselective Lewis or Brønsted acid promoted polarized [2+2] cycloadditions, see
- 26aF. de Nanteuil, J. Waser, Angew. Chem. Int. Ed. 2013, 52, 9009–9013; Angew. Chem. 2013, 125, 9179–9183;
- 26bK. Inanaga, K. Takasu, M. Ihara, J. Am. Chem. Soc. 2005, 127, 3668–3669;
- 26cM. B. Boxer, H. Yamamoto, Org. Lett. 2005, 7, 3127–3129.
- 27Y. Hayashi, K. Narasaka, Chem. Lett. 1989, 793–796.
- 28
- 28aK. Narasaka, K. Hayashi, Y. Hayashi, Tetrahedron 1994, 50, 4529–4542;
- 28bK. Narasaka, Y. Hayashi, H. Shimadzu, S. Niihata, J. Am. Chem. Soc. 1992, 114, 8869–8885;
- 28cY. Hayashi, K. Otaka, N. Saito, K. Narasaka, Bull. Chem. Soc. Jpn. 1991, 64, 2122–2127;
- 28dK. Narasaka, H. Kusama, Y. Hayashi, Bull. Chem. Soc. Jpn. 1991, 64, 1471–1478;
- 28eY. Hayashi, S. Niihata, K. Narasaka, Chem. Lett. 1990, 2091–2094;
- 28fY. Hayashi, K. Narasaka, Chem. Lett. 1990, 1295–1298;
- 28gY.-I. Ichikawa, A. Narita, A. Shiozawa, Y. Hayashi, K. Narasaka, J. Chem. Soc. Chem. Commun. 1989, 1919–1921.
- 29Engler and co-workers demonstrated that stoichiometric quantities of a Ti/TADDOL complex can promote [2+2] cycloaddition between quinones and stryrenes. T. A. Engler, M. A. Letavic, R. Iyengar, K. O. LaTessa, J. P. Reddy, J. Org. Chem. 1999, 64, 2391–2405.
- 30
- 30aH. Ito, M. Hasegawa, Y. Takenaka, T. Kobayashi, K. Iguchi, J. Am. Chem. Soc. 2004, 126, 4520–4521;
- 30bY. Takenaka, H. Ito, M. Hasegawa, K. Iguchi, Tetrahedron 2006, 62, 3380–3388;
- 30cY. Takenaka, H. Ito, K. Iguchi, Tetrahedron 2007, 63, 510–513.
- 31
- 31aE. Canales, E. J. Corey, J. Am. Chem. Soc. 2007, 129, 12686–12687; For a highlight, see:
- 31bH. Butenschön, Angew. Chem. Int. Ed. 2008, 47, 3492–3495; Angew. Chem. 2008, 120, 3544–3547.
- 32K. Ishihara, M. Fushimi, J. Am. Chem. Soc. 2008, 130, 7532–7533.
- 33J. Ficini, Tetrahedron 1976, 32, 1449–1486.
- 34
- 34aC. Schotes, R. Bigler, A. Mezzetti, Synthesis 2012, 44, 513–526;
- 34bC. Schotes, A. Mezzetti, Angew. Chem. Int. Ed. 2011, 50, 3072–3074; Angew. Chem. 2011, 123, 3128–3130.
- 35K. Enomoto, H. Oyama, M. Nakada, Chem. Eur. J. 2015, 21, 2798–2802.
- 36For a related non-enantioselective copper-catalyzed Ficini reaction, see: H. Li, R. P. Hsung, K. A. DeKorver, Y. Wei, Org. Lett. 2010, 12, 3780–3783.
- 37
- 37aM. L. Conner, Y. Xu, M. K. Brown, J. Am. Chem. Soc. 2015, 137, 3482–3485; For related non-enantioselective aluminum-promoted reactions, see:
- 37bB. B. Snider, E. Ron, J. Org. Chem. 1986, 51, 3643–3652;
- 37cH. Hoffmann, Z. M. Ismail, A. Weber, Tetrahedron Lett. 1981, 22, 1953–1956;
- 37dB. B. Snider, D. K. Spindell, J. Org. Chem. 1980, 45, 5017–5020.
- 38
- 38aM. E. Muratore, A. Homs, C. Obradors, A. M. Echavarren, Chem. Asian J. 2014, 9, 3066–3082;
- 38bF. López, J. L. Mascareñas, Beilstein J. Org. Chem. 2013, 9, 2250–2264.
- 39aA. Z. González, D. Benitez, E. Tkatchouk, W. A. Goddard III, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 5500–5507;
- 39bM. R. Luzung, P. Mauleón, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 12402–12403.
- 40
- 40aH. Teller, M. Corbet, L. Mantilli, G. Gopakumar, R. Goddard, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2012, 134, 15331–15342;
- 40bH. Teller, S. Flügge, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2010, 49, 1949–1953; Angew. Chem. 2010, 122, 1993–1997.
- 41
- 41aS. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio, J. M. González, Angew. Chem. Int. Ed. 2012, 51, 11552–11555; Angew. Chem. 2012, 124, 11720–11723; For related non-enantioselective [2+2] cycloadditions, see:
- 41bH. Faustino, P. Bernal, L. Castedo, F. López, J. L. Mascareñas, Adv. Synth. Catal. 2012, 354, 1658–1664;
- 41cS. Suárez-Pantiga, C. Hernández-Díaz, M. Piedrafita, E. Rubio, J. M. González, Adv. Synth. Catal. 2012, 354, 1651–1657;
- 41dX.-X. Li, L.-L. Zhu, W. Zhou, Z. Chen, Org. Lett. 2012, 14, 436–439;
- 41eP. Bernal-Albert, H. Faustino, A. Gimeno, G. Asensio, J. L. Mascareñas, F. López, Org. Lett. 2014, 16, 6196–6199; For a highlight, see:
- 41fP. Mauleón, ChemCatChem 2013, 5, 2149–2151.
- 42M. Jia, M. Monari, Q.-Q. Yang, M. Bandini, Chem. Commun. 2015, 51, 2320–2323.
- 43
- 43aA. Sakakura, K. Ishihara, Bull. Chem. Soc. Jpn. 2010, 83, 313–322;
- 43bK. Ishihara, K. Nakano, J. Am. Chem. Soc. 2007, 129, 8930–8931.
- 44G.-J. Duan, J.-B. Ling, W.-P. Wang, Y.-C. Luo, P.-F. Xu, Chem. Commun. 2013, 49, 4625–4627.
- 45
- 45aŁ. Albrecht, G. Dickmeiss, F. C. Acosta, C. Rodríguez-Escrich, R. L. Davis, K. A. Jørgensen, J. Am. Chem. Soc. 2012, 134, 2543–2546; For a highlight, see:
- 45bA. Parra, S. Reboredo, J. Alemán, Angew. Chem. Int. Ed. 2012, 51, 9734–9736; Angew. Chem. 2012, 124, 9872–9874.
- 46G. Talavera, E. Reyes, J. L. Vicario, L. Carrillo, Angew. Chem. Int. Ed. 2012, 51, 4104–4107; Angew. Chem. 2012, 124, 4180–4183.
- 47For mechanistic studies which point to the potential for [2+2] cycloaddition under enamine catalysis, see:
- 47aJ. Burés, A. Armstrong, D. G. Blackmond, J. Am. Chem. Soc. 2012, 134, 6741–6750;
- 47bK. Patora-Komisarska, M. Benohoud, H. Ishikawa, D. Seebach, Y. Hayashi, Helv. Chim. Acta 2011, 94, 719–745;
- 47cJ. Burés, A. Armstrong, D. G. Blackmond, J. Am. Chem. Soc. 2011, 133, 8822–8825.
- 48L.-W. Qi, Y. Yang, Y.-Y. Gui, Y. Zhang, F. Chen, F. Tian, L. Peng, L.-X. Wang, Org. Lett. 2014, 16, 6436–6439.
- 49K. S. Halskov, F. Kniep, V. H. Lauridsen, E. H. Iversen, B. S. Donslund, K. A. Jørgensen, J. Am. Chem. Soc. 2015, 137, 1685–1691.
- 50T. Shibata, K. Takami, A. Kawachi, Org. Lett. 2006, 8, 1343–1345.
- 51B.-M. Fan, X.-J. Li, F.-Z. Peng, H.-B. Zhang, A. S. C. Chan, Z.-H. Shao, Org. Lett. 2010, 12, 304–306.
- 52aQ. Yang, L. Yu, J. Xu, S. Li, S. Liu, H. Chen, Y. Zhou, L. Wang, B. Fan, Tetrahedron: Asymmetry 2014, 25, 957–961;
- 52bJ. Hu, Q. Yang, L. Yu, J. Xu, S. Liu, C. Huang, L. Wang, Y. Zhou, B. Fan, Org. Biomol. Chem. 2013, 11, 2294–2301.
- 53For selected examples, see:
- 53aN. N. Noucti, E. J. Alexanian, Angew. Chem. Int. Ed. 2015, 54, 5447–5450; Angew. Chem. 2015, 127, 5537–5540;
- 53bJ. M. Hoyt, K. T. Sylvester, S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2013, 135, 4862–4877;
- 53cK. Sakai, T. Kochi, F. Kakiuchi, Org. Lett. 2013, 15, 1024–1027;
- 53dA. Nishimura, M. Ohashi, S. Ogoshi, J. Am. Chem. Soc. 2012, 134, 15692–15695.
- 54For a recent review, see:
- 54aR. L. Danheiser, Science of Synthesis: Compounds with Four and Three Carbon Heteroatom Bonds, Vol. 23, Georg Thieme, Stuttgart, Germany, 2006; For Lewis acid promoted variants, see:
- 54bC. M. Rasik, M. K. Brown, Angew. Chem. Int. Ed. 2014, 53, 14522–14526; Angew. Chem. 2014, 126, 14750–14754;
- 54cC. M. Rasik, Y. J. Hong, D. J. Tantillo, M. K. Brown, Org. Lett. 2014, 16, 5168–5171;
- 54dC. Rasik, M. Brown, Synlett 2014, 25, 760–765;
- 54eC. M. Rasik, M. K. Brown, J. Am. Chem. Soc. 2013, 135, 1673–1676.
- 55For examples of enantioselective [2+2] cycloaddition with chiral keteniminium ions, see:
- 55aL.-Y. Chen, L. Ghosez, Tetrahedron Lett. 1990, 31, 4467–4470;
- 55bC. Houge, A. M. Frisque-Hesbain, A. Mockel, L. Ghosez, J. P. Declercq, G. Germain, M. Van Meerssche, J. Am. Chem. Soc. 1982, 104, 2920–2921.