Simple Sulfinate Synthesis Enables CH Trifluoromethylcyclopropanation†
Ryan Gianatassio
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Shuhei Kawamura
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorCecil L Eprile
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Klement Foo
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorJason Ge
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Aaron C. Burns
Department of Chemistry, Pfizer Pharmaceuticals, La Jolla Laboratories, 10770 Science Center Drive, La Jolla, CA 92121 (USA)
Search for more papers by this authorDr. Michael R. Collins
Department of Chemistry, Pfizer Pharmaceuticals, La Jolla Laboratories, 10770 Science Center Drive, La Jolla, CA 92121 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Phil S. Baran
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorRyan Gianatassio
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Shuhei Kawamura
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorCecil L Eprile
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Klement Foo
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorJason Ge
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorDr. Aaron C. Burns
Department of Chemistry, Pfizer Pharmaceuticals, La Jolla Laboratories, 10770 Science Center Drive, La Jolla, CA 92121 (USA)
Search for more papers by this authorDr. Michael R. Collins
Department of Chemistry, Pfizer Pharmaceuticals, La Jolla Laboratories, 10770 Science Center Drive, La Jolla, CA 92121 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Phil S. Baran
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorFinancial support for this work was provided by the NIH/NIGMS (GM-106210), Sigma–Aldrich, Inc., Pfizer, Inc., Uehara Memorial Foundation (postdoctoral fellowship for S.K.), and A*STAR (predoctoral fellowship for K.F.). We are grateful to Prof. A. L. Rheingold and Dr. C. E. Moore (UCSD) for X-ray crystallographic analysis, Dr. D.-H. Huang and Dr. L. Pasternack for assistance with NMR spectroscopy, and M. A. Ornelas for technical assistance.
Graphical Abstract
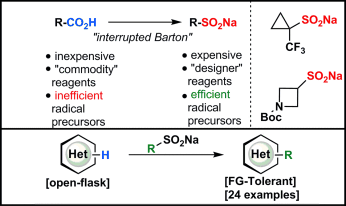
An interrupted Barton decarboxylation reaction has been used to convert readily available carboxylic acids into sulfinate salts (see scheme). Ten new sulfinate reagents were created and the reactivity of six of them towards CH functionalization was tested on a range of heterocycles. The simplicity of this approach (a cheap industrial oxidant, simple solvent, and no metals) is a clear advantage over other radical donors.
Abstract
A simple method to convert readily available carboxylic acids into sulfinate salts by employing an interrupted Barton decarboxylation reaction is reported. A medicinally oriented panel of ten new sulfinate reagents was created using this method, including a key trifluoromethylcyclopropanation reagent, TFCS-Na. The reactivity of six of these salts towards CH functionalization was field-tested using several different classes of heterocycles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406622_sm_miscellaneous_information.pdf8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond, P. S. Baran, Proc. Natl. Acad. Sci. USA 2011, 108, 14411–14415;
- 1bY. Fujiwara, J. A. Dixon, R. A. Rodriguez, R. D. Baxter, D. D. Dixon, M. R. Collins, D. G. Blackmond, P. S. Baran, J. Am. Chem. Soc. 2012, 134, 1494–1497;
- 1cY. Fujiwara, J. A. Dixon, F. O’Hara, E. Daa Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara, P. S. Baran, Nature 2012, 492, 95–99;
- 1dQ. Zhou, A. Ruffoni, R. Gianatassio, Y. Fujiwara, E. Sella, D. Shabat, P. S. Baran, Angew. Chem. 2013, 125, 4041–4044; Angew. Chem. Int. Ed. 2013, 52, 3949–3952.
- 2F. O’Hara, A. C. Burns, M. R. Collins, D. Dalvie, M. A. Ornelas, A. D. N. Vaz, Y. Fujiwara, P. S. Baran, J. Med. Chem. 2014, 57, 1616–1620.
- 3Q. Zhou, J. Gui, C.-M. Pan, E. Albone, X. Cheng, E. M. Suh, L. Grasso, Y. Ishihara, P. S. Baran, J. Am. Chem. Soc. 2013, 135, 12994–12997.
- 4E. P. Stout, M. Choi, J. E. Castro, T. F. Molinski, J. Med. Chem. 2014, 57, 5085–5093.
- 5
- 5a Bond Dissociation Energies in CRC Handbook of Chemistry and Physics, 95edth ed(Internet Version 2015), (Ed.: W. M. Haynes), CRC Press/Taylor and Francis, Boca Raton, FL, 2015;
- 5bA. Good, J. C. J. Thynne, Trans. Faraday Soc. 1967, 63, 2708–2720.
- 6D. Barnes-Seeman, M. Jain, L. Bell, S. Ferreira, S. Cohen, X. H. Chen, J. Amin, B. Snodgrass, P. Hatsis, ACS Med. Chem. Lett. 2013, 4, 514–516.
- 7
- 7aD. H. R. Barton, D. Crich, W. B. Motherwell, J. Chem. Soc. Chem. Commun. 1983, 939–941;
- 7bD. H. R. Barton, G. Kretzschmar, Tetrahedron Lett. 1983, 24, 5889–5892;
- 7cD. H. R. Barton, D. Crich, G. Kretzschmar, Tetrahedron Lett. 1984, 25, 1055–1058;
- 7dD. H. R. Barton, D. Crich, P. Potier, Tetrahedron Lett. 1985, 26, 5943–5946;
- 7eD. H. R. Barton, D. Crich, W. B. Motherwell, Tetrahedron 1985, 41, 3901–3924;
- 7fD. H. R. Barton, D. Bridon, S. Z. Zard, Heterocycles 1987, 25, 449–462;
- 7gD. H. R. Barton, D. Bridon, I. Fernandaz-Picot, S. Z. Zard, Tetrahedron 1987, 43, 2733–2740;
- 7hD. H. R. Barton, B. Lacher, B. Misterkiewicz, S. Z. Zard, Tetrahedron 1988, 44, 1153–1158;
- 7iD. H. R. Barton, N. Ozbalik, B. Vacher, Tetrahedron 1988, 44, 3501–3512;
- 7jD. H. R. Barton, F. Halley, N. Ozbalik, M. Schmitt, E. Young, G. Balavoine, J. Am. Chem. Soc. 1989, 111, 7144–7149;
- 7kD. H. R. Barton, J. C. Jaszberenyi, D. Tang, Tetrahedron Lett. 1993, 34, 3381–3384;
- 7lD. H. R. Barton, C.-Y. Chern, J. C. Jaszberenyi, T. Shinada, Tetrahedron Lett. 1993, 34, 6505–6508;
- 7mD. H. R. Barton, A. Gateau-Olesker, S. D. Géro, B. Lacher, C. Tachdjian, S. Z. Zard, Tetrahedron 1993, 49, 4589–4602;
- 7nD. H. R. Barton, W. Liu, Tetrahedron Lett. 1997, 38, 2431–2434;
- 7oD. H. R. Barton, M. Ramesh, Tetrahedron Lett. 1990, 31, 949–952;
- 7pK. U. Ingold, J. Lusztyk, B. Maillard, J. C. Walton, Tetrahedron Lett. 1988, 29, 917–920;
- 7qK. Gawronska, J. Gawronski, H. M. Walborsky, J. Org. Chem. 1991, 56, 2193–2197.
- 8
- 8aE. Castagnino, S. Corsano, Tetrahedron Lett. 1986, 27, 6337–6338;
- 8bD. H. R. Barton, B. Garcia, H. Togo, S. Z. Zard, Tetrahedron Lett. 1986, 27, 1327–1330.
- 9It has been shown that potassium trifluoromethanesulfinate can be prepared from potassium trifluoroacetate by treatment with SO2(g) at 140 °C in DMF; see B. Besson, US2009/216036, 2009.
- 10
- 10aR. C. Desai, D. F. Gratale, W. Han, H. Koyama, E. Metzger, V. K. Lombardo, K. L. MacNaul, T. W. Doebber, J. P. Berger, K. Leung, R. Franklin, D. E. Moller, J. V. Heck, S. P. Sahoo, Bioorg. Med. Chem. Lett. 2003, 13, 3541–3544;
- 10bJ. M. Ontoria, L. L. Bufi, C. Torrisi, A. Bresciani, C. Giomini, M. Rowley, S. Serafini, H. Bin, W. Hao, C. Steinkuhler, P. Jones, Bioorg. Med. Chem. Lett. 2011, 21, 5274–5282;
- 10cL. Liu, M. H. Norman, M. Lee, N. Xi, A. Siegmund, A. A. Boezio, S. Booker, D. Choquette, N. D. D’Angelo, J. Germain, K. Yang, Y. Yang, Y. Zhang, S. F. Bellon, D. A. Whittington, J. C. Harmange, C. Dominguez, T. S. Kim, I. Dussault, J. Med. Chem. 2012, 55, 1868–1897;
- 10dS. T. Waddell, G. M. Santorelli, M. M. Maletic, A. H. Leeman, X. Gu, D. W. Graham, J. M. Balkovec, S. D. Aster, US2004/133011, 2004.
- 11R. Jain, B. Vaitilingam, A. Nayyar, P. B. Palde, Bioorg. Med. Chem. Lett. 2003, 13, 1051–1054.
- 12
- 12aZ. J. Li, Z. L. Cui, Z. Q. Liu, Org. Lett. 2013, 15, 406–409;
- 12bY. D. Ye, S. A. Kuenzi, M. S. Sanford, Org. Lett. 2012, 14, 4979–4981.
- 13
- 13aL. Alig, J. Alsenz, M. Andjelkovic, S. Bendels, A. Bernardeau, K. Bleicher, A. Bourson, P. David-Pierson, W. Guba, S. Hildbrand, D. Kube, T. Lubbers, A. V. Mayweg, R. Narquizian, W. Neidhart, M. Nettekoven, J. M. Plancher, C. Rocha, M. Rogers-Evans, S. Rover, G. Schneider, S. Taylor, P. Waldineier, J. Med. Chem. 2008, 51, 2115–2127;
- 13bM. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti, F. D. Toste, Angew. Chem. 2014, 126, 4493–4496;
10.1002/ange.201400037 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 4404–4407.
- 14J. Banville, R. Rémillard, E. H. Ruediger, D. H. Deon, M. Gagnon, L. Dúbe, J. Guy, E. S. Priestly, S. L. Posy, B. D. Maxwell, P. C. Wong, WO2013/163279, 2013.
- 15
- 15aM. Presset, N. Fleury-Bregeot, D. Oehlrich, F. Rombouts, G. A. Molander, J. Org. Chem. 2013, 78, 4615–4619;
- 15bG. A. Molander, V. Colombel, V. A. Braz, Org. Lett. 2011, 13, 1852–1855.
- 16M. S. Malmas, Y. Ni, J. J. Edrei, H. Stange, R. Schindler, N. Hofgen, U. Egerland, B. Langen, US2009/14336, 2009.
- 17R. Oballa, WO2006/133559, 2006.