Poly(dimethylsiloxane)-Supported Ionogels with a High Ionic Liquid Loading†
This research was performed with financial support from the National Science Foundation (award no. ECCS-1201935) and the US Army Natick Soldier Research, Defense and Engineering Center.
Graphical Abstract
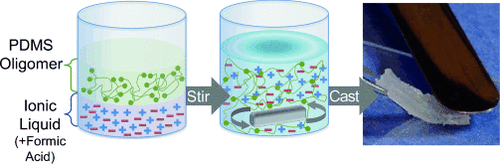
Supportive, despite their differences: The immiscibility of poly(dimethylsiloxane) (PDMS) and ionic liquids (ILs) has been overcome by a simple sol–gel reaction (see picture) to create PDMS-supported ILs (ionogels) with IL loadings of up to 80 % by mass. The ionogels exhibited high ionic conductivity and excellent mechanical behavior, with an elastic modulus of approximately 60 kPa without fatigue over 5000 cycles, even at elevated temperatures.
Abstract
The immiscibility of poly(dimethylsiloxane) (PDMS) and ionic liquids (ILs) was overcome to create PDMS-supported IL gels (ionogels) with IL loadings of up to 80 % by mass through a simple sol–gel reaction at room temperature. By stirring a mixture of a functionalized PDMS oligomer, formic acid, and an IL (or lithium-in-IL solution), a resin was formed that could be cast to create a freestanding, flexible ionogel. PDMS-supported ionogels exhibited favorable ionic conductivity (ca. 3 mS cm−1) and excellent mechanical behavior (elastic modulus: ca. 60 kPa; fatigue life: >5000 cycles; mechanically stable at temperatures up to 200 °C). The activation energy of ionic conductivity was shown to be nearly identical for the ionogel and the neat IL, in contrast to ionogel systems wherein the scaffold material is miscible with the IL. This similarity indicates that IL/scaffold chemical interactions are key to the understanding of ionogel electrical performance, especially at elevated temperatures.