Chemoselective Carbophilic Addition of α-Diazoesters through Ligand-Controlled Gold Catalysis†
Yumeng Xi
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
These authors contributed equally to this work.
Search for more papers by this authorYijin Su
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
These authors contributed equally to this work.
Search for more papers by this authorZhaoyuan Yu
School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Search for more papers by this authorBoliang Dong
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorEdward J. McClain
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorCorresponding Author
Prof. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Yu Lan, School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Xiaodong Shi, C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorCorresponding Author
Prof. Xiaodong Shi
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Yu Lan, School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Xiaodong Shi, C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorYumeng Xi
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
These authors contributed equally to this work.
Search for more papers by this authorYijin Su
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
These authors contributed equally to this work.
Search for more papers by this authorZhaoyuan Yu
School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Search for more papers by this authorBoliang Dong
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorEdward J. McClain
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorCorresponding Author
Prof. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Yu Lan, School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Xiaodong Shi, C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorCorresponding Author
Prof. Xiaodong Shi
C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Yu Lan, School of Chemistry and Chemical Engineering, Chongqing University, 400030, Chongqing (P.R. China)
Xiaodong Shi, C. Eugene Bennett Department of Chemistry, West Virginia University, Morgantown, WV 26506 (USA) http://community.wvu.edu/∼xs007/
Search for more papers by this authorWe are grateful to the NSF (CAREER-CHE-0844602 and CHE-1228336) and the NSFC (No. 21228204) for financial support. Y.L. acknowledges support from the National Science Foundation of China (No. 21372266 and 51302327).
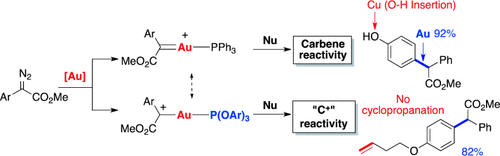
Graphical Abstract
Carbocation or carbene? The chemoselective addition of arenes and 1,3-diketones to α-aryldiazoesters was achieved through ligand-controlled gold catalysis. The gold catalyst with electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition products, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.
Abstract
The chemoselective addition of arenes and 1,3-diketones to α-aryldiazoesters was achieved through ligand-controlled gold catalysis. Unlike a dirhodium catalyst (which promotes C H insertion and cyclopropanation) and a copper catalyst (which catalyzes OH and NH insertions), the gold catalyst with an electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition product, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.
H insertion and cyclopropanation) and a copper catalyst (which catalyzes OH and NH insertions), the gold catalyst with an electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition product, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404946_sm_miscellaneous_information.pdf39.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. S. K. Hashmi, F. D. Toste, Modern Gold Catalyzed Synthesis, Wiley-VCH, Weinheim, 2012;
10.1002/9783527646869 Google Scholar
- 1bA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2012, 41, 2448;
- 1cD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351;
- 1dA. Arcadi, Chem. Rev. 2008, 108, 3266;
- 1eE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326;
- 1fD. J. Gorin, F. D. Toste, Nature 2007, 446, 395;
- 1gA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180;
- 1hA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410;
- 1iL. Zhang, J. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271;
- 1jA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. 2006, 118, 8064;
10.1002/ange.200602454 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7896.
- 2
- 2aA. Fürstner, L. Morency, Angew. Chem. 2008, 120, 5108;
10.1002/ange.200800934 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5030;
- 2bA. S. K. Hashmi, Angew. Chem. 2008, 120, 6856;
10.1002/ange.200802517 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6754;
- 2cG. Seidel, R. Mynott, A. Fürstner, Angew. Chem. 2009, 121, 2548;
10.1002/ange.200806059 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2510;
- 2dA. M. Echavarren, Nat. Chem. 2009, 1, 431;
- 2eA. Fedorov, L. Batiste, A. Bach, D. M. Birney, P. Chen, J. Am. Chem. Soc. 2011, 133, 12162–12171.
- 3
- 3aO. Nieto Faza, C. Silva López, R. Álvarez, A. R. de Lera, J. Am. Chem. Soc. 2006, 128, 2434;
- 3bA. Correa, N. Marion, L. Fensterbank, M. Malacria, S. P. Nolan, L. Cavallo, Angew. Chem. 2008, 120, 730;
10.1002/ange.200703769 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 718;
- 3cV. López-Carrillo, N. Huguet, Á. Mosquera, A. M. Echavarren, Chem. Eur. J. 2011, 17, 10972.
- 4R. E. M. Brooner, T. J. Brown, R. A. Widenhoefer, Angew. Chem. 2013, 125, 6379;
10.1002/ange.201301640 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 6259.
- 5
- 5aG. Seidel, A. Fürstner, Angew. Chem. 2014, 126, 4907;
10.1002/ange.201402080 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 4807; for related examples, see:
- 5bG. Seidel, B. Gabor, R. Goddard, B. Heggen, W. Thiel, A. Fürstner, Angew. Chem. 2014, 126, 898;
10.1002/ange.201308842 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 879;
- 5cR. E. M. Brooner, R. A. Widenhoefer, Chem. Commun. 2014, 50, 2420.
- 6While this manuscript was under review, a similar study was reported: Z. Yu, B. Ma, M. Chen, H.-H. Wu, L. Liu, J. Zhang, J. Am. Chem. Soc. 2014, 136, 6904.
- 7
- 7aM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704;
- 7bM. P. Doyle, Chem. Rev. 1986, 86, 919;
- 7cH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 7dZ. Zhang, J. Wang, Tetrahedron 2008, 64, 6577.
- 8For an instructive review, see: H. M. L. Davies, D. Mortona, Chem. Soc. Rev. 2011, 40, 1857.
- 9For an instructive review, see:
- 9aS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365–1377;
- 9bX. Zhao, Y. Zhang, J. Wang, Chem. Commun. 2012, 48, 10162.
- 10M. R. Fructos, T. R. Belderrain, P. de Frémont, N. M. Scott, S. P. Nolan, M. M. Díaz-Requejo, P. J. Pérez, Angew. Chem. 2005, 117, 5418;
10.1002/ange.200501056 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5284.
- 11
- 11aM. R. Fructos, P. de Frémont, S. P. Nolan, M. M. Díaz-Requejo, P. J. Pérez, Organometallics 2006, 25, 2237;
- 11bM. R. Fructos, M. M. Díaz-Requejo, P. J. Pérez, Chem. Commun. 2009, 5153;
- 11cA. Prieto, M. R. Fructos, M. M. Díaz-Requejo, P. J. Pérez, P. Pérez-Galán, N. Delpont, A. M. Echavarren, Tetrahedron 2009, 65, 1790;
- 11dV. V. Pagar, A. M. Jadhav, R.-S. Liu, J. Am. Chem. Soc. 2011, 133, 20728;
- 11eA. M. Jadhav, V. V. Pagar, R.-S. Liu, Angew. Chem. 2012, 124, 11979;
10.1002/ange.201205692 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11809;
- 11fJ. F. Briones, H. M. L. Davies, J. Am. Chem. Soc. 2012, 134, 11916;
- 11gS. K. Pawar, C.-D. Wang, S. Bhunia, A. M. Jadhav, R.-S. Liu, Angew. Chem. 2013, 125, 7707; Angew. Chem. Int. Ed. 2013, 52, 7559;
- 11hJ. Barluenga, G. Lonzi, M. Tomás, L. A. López, Chem. Eur. J. 2013, 19, 1573;
- 11iG. Lonzi, L. A. López, Adv. Synth. Catal. 2013, 355, 1948;
- 11jJ. F. Briones, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 13314.
- 12
- 12aH. M. L. Davies, Q. Jin, Tetrahedron: Asymmetry 2003, 14, 941;
- 12bH. M. L. Davies, Q. Jin, P. Ren, A. Y. Kovalevsky, J. Org. Chem. 2002, 67, 4165;
- 12cH. M. L. Davies, J. Yang, J. Nikolai, J. Organomet. Chem. 2005, 690, 6111;
- 12dA similar aromatic substitution with diazo compounds has been described with copper catalysts; however, only a very limited scope was reported: E. Tayama, T. Yanaki, H. Iwamoto, E. Hasegawa, Eur. J. Org. Chem. 2010, 6719.
- 13
- 13aT. C. Maier, G. C. Fu, J. Am. Chem. Soc. 2006, 128, 4594;
- 13bC. Chen, S.-F. Zhu, B. Liu, L.-X. Wang, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 12616;
- 13cX.-L. Xie, S.-F. Zhu, J.-X. Guo, Y. Cai, Q.-L. Zhou, Angew. Chem. 2014, 126, 3022; Angew. Chem. Int. Ed. 2014, 53, 2978.
- 14Y. Luo, K. Ji, Y. Li, L. Zhang, J. Am. Chem. Soc. 2012, 134, 17412.
- 15L. Zhang, Acc. Chem. Res. 2014, 47, 877.
- 16K. P. Kornecki, J. F. Briones, V. Boyarskikh, F. Fullilove, J. Autschbach, K. E. Schrote, K. M. Lancaster, H. M. L. Davies, J. F. Berry, Science 2013, 342, 351.
- 17
- 17aP. Mauleón, R. M. Zeldin, A. Z. González, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6348;
- 17bM. Alcarazo, T. Stork, A. Anoop, W. Thiel, A. Fürstner, Angew. Chem. 2010, 122, 2596; Angew. Chem. Int. Ed. 2010, 49, 2542;
- 17cI. Alonso, B. Trillo, F. López, S. Montserrat, G. Ujaque, L. Castedo, A. Lledós, J. L. Mascareñas, J. Am. Chem. Soc. 2009, 131, 13020.
- 18The typical metal-catalyzed α-diazoester decomposition usually requires strict conditions, such as anhydrous solvent, careful degassing, and syringe pump addition, to avoid side reactions (including water addition, oxidation, and dimerization).
- 19In 1,n-enyne cycloisomerization reactions, the proposed gold–carbenoid could be intercepted by a carbon nucleophile: C. H. M. Amijs, V. López-Carrillo, M. Raducan, P. Pérez-Galán, C. Ferrer, A. M. Echavarren, J. Org. Chem. 2008, 73, 7721.
- 20See Ref. [12] and [13].
- 21B. Liu, S.-F. Zhu, W. Zhang, C. Chen, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 5834.
- 22S. J. Hedley, D. L. Ventura, P. M. Dominiak, C. L. Nygren, H. M. L. Davies, J. Org. Chem. 2006, 71, 5349.
- 23H. M. Mbuvi, L. K. Woo, J. Porphyrins Phthalocyanines 2009, 13, 136.
- 24See Ref. [11f] and [11c].
- 25Z.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou, K. Ding, J. Am. Chem. Soc. 2013, 135, 8197.
- 26
- 26aC. H. M. Amijs, V. López-Carrillo, A. M. Echavarren, Org. Lett. 2007, 9, 4021;
- 26bY. Xi, D. Wang, X. Ye, N. G. Akhmedov, J. L. Petersen, X. Shi, Org. Lett. 2014, 16, 306.
- 27W. Zhao, D. M. Fink, C. A. Labutta, A. T. Radosevich, Org. Lett. 2013, 15, 3090.
- 28
- 28aRef. [25b];
- 28bY. Xi, B. Dong, X. Shi, Beilstein J. Org. Chem. 2013, 9, 2537.
- 29For a theoretical study on ligand effects in gold–carbenoid chemistry, see: D. Benitez, N. D. Shapiro, E. Tkatchouk, Y. Wang, W. A. Goddard III, F. D. Toste, Nat. Chem. 2009, 1, 482.
- 30R. Peverati, D. G. Truhlar, J. Phys. Chem. Lett. 2011, 2, 2810.
- 31
- 31aP. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 270;
- 31bP. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 284;
- 31cP. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 299.
- 32Calculation results obtained with other DFT methods are summarized in the Supporting Information.
- 33It is not clear at this moment whether this 1,2-proton shift is concerted or stepwise.