Synthesis of the Heparin-Based Anticoagulant Drug Fondaparinux†
Dr. Cheng-Hsiu Chang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Department of Chemistry, National Tsing Hua University, No. 101, Section 2, Kuang-Fu Road, Hsinchu 300 (Taiwan)
Search for more papers by this authorLarry S. Lico
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Institute of Chemistry, University of the Philippines, Diliman, Quezon City 1101 (Philippines)
Search for more papers by this authorDr. Teng-Yi Huang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorDr. Shu-Yi Lin
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorDr. Chi-Liang Chang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorProf. Dr. Susan D. Arco
Institute of Chemistry, University of the Philippines, Diliman, Quezon City 1101 (Philippines)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shang-Cheng Hung
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)Search for more papers by this authorDr. Cheng-Hsiu Chang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Department of Chemistry, National Tsing Hua University, No. 101, Section 2, Kuang-Fu Road, Hsinchu 300 (Taiwan)
Search for more papers by this authorLarry S. Lico
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Institute of Chemistry, University of the Philippines, Diliman, Quezon City 1101 (Philippines)
Search for more papers by this authorDr. Teng-Yi Huang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorDr. Shu-Yi Lin
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorDr. Chi-Liang Chang
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Search for more papers by this authorProf. Dr. Susan D. Arco
Institute of Chemistry, University of the Philippines, Diliman, Quezon City 1101 (Philippines)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shang-Cheng Hung
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)
Genomics Research Center, Academia Sinica, No. 128 Academia Road, Section 2, Taipei 115 (Taiwan)Search for more papers by this authorThis work was supported by the Ministry of Science and Technology (NSC 100-2113-M-001-019-MY3 and NSC 101-2628-M-001-006-MY3), National Health Research Institutes (NHRI-EX101-10146NI), and Academia Sinica.
Graphical Abstract
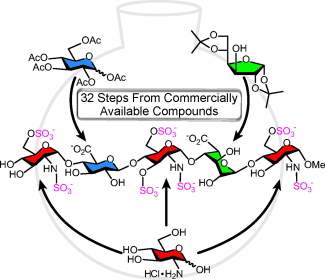
Working against the clot: The synthetic anticoagulant fondaparinux, a pentasaccharide based on the antithrombin-binding domain of heparin, was prepared in a concise and efficient manner in the shortest route reported to date. The application of one-pot strategies, the use of common intermediates, and the efficient preparation of monosaccharide building blocks from commercial sources are key features of this approach.
Abstract
Fondaparinux, a synthetic pentasaccharide based on the heparin antithrombin-binding domain, is an approved clinical anticoagulant. Although it is a better and safer alternative to pharmaceutical heparins in many cases, its high cost, which results from the difficult and tedious synthesis, is a deterrent for its widespread use. The chemical synthesis of fondaparinux was achieved in an efficient and concise manner from commercially available D-glucosamine, diacetone α-D-glucose, and penta-O-acetyl-D-glucose. The method involves suitably functionalized building blocks that are readily accessible and employs shared intermediates and a series of one-pot reactions that considerably reduce the synthetic effort and improve the yield.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404154_sm_miscellaneous_information.pdf6.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aI. Capila, R. J. Linhardt, Angew. Chem. 2002, 114, 426–450;
10.1002/1521-3757(20020201)114:3<426::AID-ANGE426>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2002, 41, 390–412;
- 1bR. J. Linhardt, J. Med. Chem. 2003, 46, 2551–2564.
- 2
- 2aC. A. A. van Boeckel, M. Petitou, Angew. Chem. 1993, 105, 1741–1761; Angew. Chem. Int. Ed. Engl. 1993, 32, 1671–1690;
- 2bM. Petitou, B. Casu, U. Lindahl, Biochimie 2003, 85, 83–89.
- 3J. I. Weitz, L.-A. Linkins, Expert Opin. Invest. Drugs 2007, 16, 271–282.
- 4D. B. Blossom, A. J. Kallen, P. R. Patel, A. Elward, L. Robinson, G. Gao, R. Langer, K. M. Perkins, J. L. Jaeger, K. M. Kurkjian, M. Jones, S. F. Schillie, N. Shehab, D. Ketterer, G. Venkataraman, T. K. Kishimoto, Z. Shriver, A. W. McMahon, K. F. Austen, S. Kozlowski, A. Srinivasan, G. Turabelidze, C. V. Gould, M. J. Arduino, R. Sasisekharan, N. Engl. J. Med. 2008, 359, 2674–2684.
- 5M. Petitou, C. A. A. van Boeckel, Angew. Chem. 2004, 116, 3180–3196;
10.1002/ange.200300640 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3118–3133.
- 6
- 6aJ. M. Walenga, W. P. Jeske, F. X. Frapaise, R. L. Bick, J. Fareed, M. M. Samama, Expert Opin. Invest. Drugs 2002, 11, 397–407;
- 6bS. Middeldorp, Thromb. Res. 2008, 122, 753–762;
- 6cM. Nagler, M. Haslauer, W. A. Wuillemin, Thromb. Res. 2012, 129, 407–417.
- 7
- 7aC. A. A. van Boeckel, T. Beetz, J. N. Vos, A. J. M. de Jong, S. F. van Aelst, R. H. van den Bosch, J. M. R. Mertens, F. A. van der Vlugt, J. Carbohydr. Chem. 1985, 4, 293–321;
- 7bM. Petitou, J.-C. Jacquinet, P. Sinay, J. Choay, J.-C. Lorraeau, M. Nassr, US Patent 4,818,816, 1989.
- 8
- 8aC. Noti, J. L. de Paz, L. Polito, P. H. Seeberger, Chem. Eur. J. 2006, 12, 8664–8686;
- 8bF. Baleux, L. Loureiro-Morais, Y. Hersant, P. Clayette, F. Arenzana-Seisdedos, D. Bonnaffé, H. Lortat-Jacob, Nat. Chem. Biol. 2009, 5, 743–748;
- 8cS. Arungundram, K. Al-Mafraji, J. Asong, F. E. Leach III, I. J. Amster, A. Venot, J. E. Turnbull, G.-J. Boons, J. Am. Chem. Soc. 2009, 131, 17394–17405;
- 8dZ. Wang, Y. Xu, B. Yang, G. Tiruchinapally, B. Sun, R. Liu, S. Dulaney, J. Liu, X. Huang, Chem. Eur. J. 2010, 16, 8365–8375;
- 8eS. U. Hansen, G. J. Miller, C. Cole, G. Rushton, E. Avizienyte, G. C. Jayson, J. M. Gardiner, Nat. Commun. 2013, 4, 2016;
- 8fM. M. L. Zulueta, S.-Y. Lin, S.-C. Hung, Trends Glycosci. Glycotechnol. 2013, 25, 141–158;
- 8gM. M. L. Zulueta, S.-Y. Lin, Y.-P. Hu, S.-C. Hung, Curr. Opin. Chem. Biol. 2013, 17, 1023–1029.
- 9
- 9aY. Xu, S. Masuko, M. Takieddin, H. Xu, R. Liu, J. Jing, S. A. Mousa, R. J. Linhardt, J. Liu, Science 2011, 334, 498–501;
- 9bF. Lin, G. Lian, Y. Zhou, Carbohydr. Res. 2013, 371, 32–39;
- 9cR. Kovi, A. Naik, B. Patel, M. Madala (Apicore), WO 2013/003001 A1, 2013;
- 9dP. P. Patel, C. Ma, K. K. Ohrr, S. Nadji (Reliable Pharmaceutical), WO 2013/115817 A1, 2013;
- 9eT. Li, H. Ye, X. Cao, J. Wang, Y. Liu, L. Zhou, Q. Liu, W. Wang, J. Shen, W. Zhao, P. Wang, ChemMedChem 2014, 9, 1071–1080.
- 10
- 10aC.-C. Wang, J.-C. Lee, S.-Y. Luo, S. S. Kulkarni, Y.-W. Huang, C.-C. Lee, K.-L. Chang, S.-C. Hung, Nature 2007, 446, 896–899;
- 10bC.-C. Wang, S. S. Kulkarni, J.-C. Lee, S.-Y. Luo, S.-C. Hung, Nat. Protoc. 2008, 3, 97–113;
- 10cT.-Y. Huang, M. M. L. Zulueta, S.-C. Hung, Org. Lett. 2011, 13, 1506–1509;
- 10dT.-Y. Huang, M. M. L. Zulueta, S.-C. Hung, Org. Biomol. Chem. 2014, 12, 376–382.
- 11
- 11aY.-P. Hu, S.-Y. Lin, C.-Y. Huang, M. M. L. Zulueta, J.-Y. Liu, W. Chang, S.-C. Hung, Nat. Chem. 2011, 3, 557–563;
- 11bC.-C. Chung, M. M. L. Zulueta, L. T. Padiyar, S.-C. Hung, Org. Lett. 2011, 13, 5496–5499;
- 11cM. M. L. Zulueta, S.-Y. Lin, Y.-T. Lin, C.-J. Huang, C.-C. Wang, C.-C. Ku, Z. Shi, C.-L. Chyan, D. Irene, L.-H. Lim, T.-I. Tsai, Y.-P. Hu, S. D. Arco, C.-H. Wong, S.-C. Hung, J. Am. Chem. Soc. 2012, 134, 8988–8995.
- 12
- 12aS.-C. Hung, X.-A. Lu, J.-C. Lee, M. D.-T. Chang, S.-l. Fang, T.-c. Fan, M. M. L. Zulueta, Y.-Q. Zhong, Org. Biomol. Chem. 2012, 10, 760–772;
- 12bY.-P. Hu, Y.-Q. Zhong, Z.-G. Chen, C.-Y. Chen, Z. Shi, M. M. L. Zulueta, C.-C. Ku, P.-Y. Lee, C.-C. Wang, S.-C. Hung, J. Am. Chem. Soc. 2012, 134, 20722–20727.
- 13L.-D. Lu, C.-R. Shie, S. S. Kulkarni, G.-R. Pan, X.-A. Lu, S.-C. Hung, Org. Lett. 2006, 8, 5995–5998.
- 14A. A. Joseph, V. P. Verma, X.-Y. Liu, C.-H. Wu, V. M. Dhurandhare, C.-C. Wang, Eur. J. Org. Chem. 2012, 744–753.
- 15P. Angibeaud, J.-P. Utille, Synthesis 1991, 737–738.