Oxidative Catalysis Using the Stoichiometric Oxidant as a Reagent: An Efficient Strategy for Single-Electron-Transfer-Induced Tandem Anion–Radical Reactions†
Ing. František Kafka
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
These authors contributed equally to this work.
Search for more papers by this authorIng. Martin Holan
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
These authors contributed equally to this work.
Search for more papers by this authorM.Sc. Denisa Hidasová
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorDr. Radek Pohl
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorDr. Ivana Císařová
Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030/8, 12843 Prague (Czech Republic)
Search for more papers by this authorDr. Blanka Klepetářová
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorCorresponding Author
Dr. Ullrich Jahn
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.htmlSearch for more papers by this authorIng. František Kafka
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
These authors contributed equally to this work.
Search for more papers by this authorIng. Martin Holan
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
These authors contributed equally to this work.
Search for more papers by this authorM.Sc. Denisa Hidasová
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorDr. Radek Pohl
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorDr. Ivana Císařová
Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030/8, 12843 Prague (Czech Republic)
Search for more papers by this authorDr. Blanka Klepetářová
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorCorresponding Author
Dr. Ullrich Jahn
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.html
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic) http://www.uochb.cz/web/structure/616.htmlSearch for more papers by this authorGenerous financial support by the Grant Agency of the Czech Republic (13-40188S), the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic (RVO: 61388963), the COST action CM1201 “Biomimetic Radical Chemistry”, and the Gilead Sciences & IOCB Research Center is gratefully acknowledged. I.C. thanks the Ministry of Education, Youth and Sports of the Czech Republic (MSM0021620857) for financial support. We thank Prof. Dr. Klaus Dietrich (BASF SE) for a gift of the 1-phenylethylamine enantiomers.
Graphical Abstract
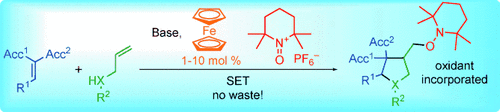
Making waste a functionality: Oxidative electron-transfer-mediated anion–radical transformations are rendered catalytic by employing a 2,2,6,6-tetramethyl-N-oxopiperidinium salt and ferrocene. The method provides an asymmetric approach to highly functionalized cyclopentane and pyrrolidine derivatives. At the same time the co-generated reduced species TEMPO serves as a useful oxygenating functionality.
Abstract
Oxidative single-electron transfer-catalyzed tandem reactions consisting of a conjugate addition and a radical cyclization are reported, which incorporate the mandatory terminal oxidant as a functionality into the product.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403776_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aU. Jahn, Top. Curr. Chem. 2012, 320, 121–190;
- 1bU. Jahn, Top. Curr. Chem. 2012, 320, 191–322;
- 1cU. Jahn, Top. Curr. Chem. 2012, 320, 323–452.
- 2
- 2aX. Hu, Chem. Sci. 2011, 2, 1867–1886;
- 2bS. E. Denmark, A. J. Cresswell, J. Org. Chem. 2013, 78, 12593–12628.
- 3
- 3aJ. M. Muñoz-Molina, T. R. Belderrain, P. J. Pérez, Eur. J. Inorg. Chem. 2011, 3155–3164;
- 3bW. T. Eckenhoff, T. Pintauer, Catal. Rev. 2010, 52, 1–59;
- 3cK. Severin, Curr. Org. Chem. 2006, 10, 217–224.
- 4Selected review: A. Studer, D. P. Curran, Angew. Chem. 2011, 123, 5122–5127; Angew. Chem. Int. Ed. 2011, 50, 5018–5022.
- 5Selected recent reviews:
- 5aJ. A. Murphy in Encyclopedia of Radicals in Chemistry, Biology and Materials, Vol. 2 (Eds.: ), Wiley, Hoboken, 2012, pp. 817;
- 5bD. J. Procter, R. A. Flowers II, T. Skrydstrup, Organic Synthesis Using Samarium Diiodide, RSC, Cambridge, 2009;
- 5cJ. Justicia, L. A. de Cienfuegos, A. G. Campaña, D. Miguel, V. Jakoby, A. Gansäuer, J. M. Cuerva, Chem. Soc. Rev. 2011, 40, 3525–3537.
- 6
- 6aJ. W. Burton in Encyclopedia of Radicals in Chemistry, Biology and Materials, Vol. 2 (Eds.: ), Wiley, Hoboken, 2012, pp. 901–941;
- 6bS. E. Allen, R. R. Walvoord, R. Padilla-Salinas, M. C. Kozlowski, Chem. Rev. 2013, 113, 6234–6458;
- 6cF. H. Guo, M. D. Clift, R. J. Thomson, Eur. J. Org. Chem. 2012, 4881–4896;
- 6dZ. Shi, C. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3381–3430.
- 7A. Gansäuer, J. Justicia, C. A. Fan, D. Worgull, F. Piestert, Top. Curr. Chem. 2007, 279, 25–52, and reviews therein.
- 8Selected recent reviews:
- 8aC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322–5363;
- 8bM. Majek, A. J. von Wangelin, Angew. Chem. 2013, 125, 6033–6035;
10.1002/ange.201301843 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 5919–5921;
- 8cT. P. Yoon, ACS Catal. 2013, 3, 895–902;
- 8dY. Xi, H. Yi, A. Lei, Org. Biomol. Chem. 2013, 11, 2387–2403;
- 8eJ. Xuan, W.-J. Xiao, Angew. Chem. 2012, 124, 6934–6944; Angew. Chem. Int. Ed. 2012, 51, 6828–6838;
- 8fJ. W. Tucker, C. R. J. Stephenson, J. Org. Chem. 2012, 77, 1617–1622;
- 8gF. Teplý, Collect. Czech. Chem. Commun. 2011, 76, 859–917.
- 9For a recent Highlight, from which the sacrificial oxidant problems can be derived: Q. Liu, R. Jackstell, M. Beller, Angew. Chem. 2013, 125, 14115–14117; Angew. Chem. Int. Ed. 2013, 52, 13871–13873.
- 10Selected reviews:
- 10aJ. F. Bower, I. S. Kim, R. L. Patman, M. J. Krische, Angew. Chem. 2009, 121, 36–48; Angew. Chem. Int. Ed. 2009, 48, 34–46;
- 10bG. E. Dobereiner, R. H. Crabtree, Chem. Rev. 2010, 110, 681–703;
- 10cS. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann, M. Beller, ChemCatChem 2011, 3, 1853–1864;
- 10dJ. Schranck, A. Tlili, M. Beller, Angew. Chem. 2013, 125, 7795–7797;
10.1002/ange.201303015 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7642–7644.
- 11
- 11aU. Jahn, F. Kafka, R. Pohl, P. G. Jones, Tetrahedron 2009, 65, 10917–10929;
- 11bE. Dinca, P. Hartmann, J. Smrček, I. Dix, P. G. Jones, U. Jahn, Eur. J. Org. Chem. 2012, 4461–4482.
- 12Recent review: S. G. Davies, A. M. Fletcher, P. M. Roberts, J. E. Thomson, Tetrahedron: Asymmetry 2012, 23, 1111–1153.
- 13U. Jahn, M. Müller, S. Aussieker, J. Am. Chem. Soc. 2000, 122, 5212–5213.
- 14Selected examples:
- 14aU. Jahn, E. Dinca, Chem. Eur. J. 2009, 15, 58–62;
- 14bU. Jahn, E. Dinca, J. Org. Chem. 2010, 75, 4480–4491;
- 14cJ. Xu, E. J. E. Caro-Diaz, L. Trzoss, E. A. Theodorakis, J. Am. Chem. Soc. 2012, 134, 5072–5075.
- 15Selected references:
- 15aM. Schämann, H. J. Schäfer, Synlett 2004, 1601–1603, and references therein;
- 15bH. Richter, R. Fröhlich, C.-G. Daniliuc, O. G. Mancheño, Angew. Chem. 2012, 124, 8784–8788; Angew. Chem. Int. Ed. 2012, 51, 8656–8660;
- 15cH. Richter, O. G. Mancheño, Org. Lett. 2011, 13, 6066–6069;
- 15dX.-Y. Duan, N.-N. Zhou, R. Fang, X.-L. Yang, W. Yu, B. Han, Angew. Chem. 2014, 126, 3222–3226; Angew. Chem. Int. Ed. 2014, 53, 3158–3162.
- 16M. Holan, R. Pohl, I. Císařová, U. Jahn, Eur. J. Org. Chem. 2012, 3459–3475.