Ligand Modification Transforms a Catalase Mimic into a Water Oxidation Catalyst†
Dr. Wei-Tsung Lee
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorSalvador B. Muñoz III
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorDr. Diane A. Dickie
Department of Chemistry and Chemical Biology, The University of New Mexico, Albuquerque, NM 87131 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jeremy M. Smith
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)Search for more papers by this authorDr. Wei-Tsung Lee
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorSalvador B. Muñoz III
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Search for more papers by this authorDr. Diane A. Dickie
Department of Chemistry and Chemical Biology, The University of New Mexico, Albuquerque, NM 87131 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jeremy M. Smith
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)
Department of Chemistry, Indiana University, 800 East Kirkwood Ave., Bloomington, IN 47405 (USA)Search for more papers by this authorFunding from Indiana University, the American Chemical Society (Petroleum Research Foundation, 50971-ND3), and the U.S. Department of Energy (Office of Basic Energy Sciences; DE-FG02-08ER15996) is gratefully acknowledged. J.M.S. is a Dreyfus Teacher-Scholar. S.B.M. acknowledges support from NIH-RISE (R25 GM06122211). We thank Song Xu, Dennis Chen, and Sara Skrabalak for experimental assistance.
Graphical Abstract
Abstract
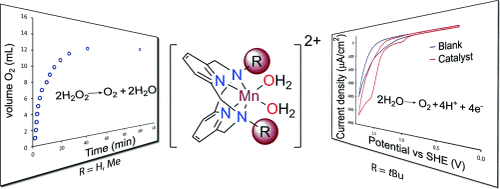
The catalytic reactivity of the high-spin MnII pyridinophane complexes [(Py2NR2)Mn(H2O)2]2+ (R=H, Me, tBu) toward O2 formation is reported. With small macrocycle N-substituents (R=H, Me), the complexes catalytically disproportionate H2O2 in aqueous solution; with a bulky substituent (R=tBu), this catalytic reaction is shut down, but the complex becomes active for aqueous electrocatalytic H2O oxidation. Control experiments are in support of a homogeneous molecular catalyst and preliminary mechanistic studies suggest that the catalyst is mononuclear. This ligand-controlled switch in catalytic reactivity has implications for the design of new manganese-based water oxidation catalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402407_sm_miscellaneous_information.pdf1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. J. Wu, J. E. Penner-Hahn, V. L. Pecoraro, Chem. Rev. 2004, 104, 903–938.
- 2
- 2aS. V. Antonyuk, V. R. Melik-Adamyan, A. N. Popov, V. S. Lamzin, P. D. Hempstead, P. M. Harrison, P. J. Artymyuk, V. V. Barynin, Crystallogr. Rep. 2000, 45, 105–116;
- 2bV. V. Barynin, M. M. Whittaker, S. V. Antonyuk, V. S. Lamzin, P. M. Harrison, P. J. Artymiuk, J. W. Whittaker, Structure 2001, 9, 725–738.
- 3
- 3aJ. P. McEvoy, G. W. Brudvig, Chem. Rev. 2006, 106, 4455–4483;
- 3bH. Dau, I. Zaharieva, M. Haumann, Curr. Opin. Chem. Biol. 2012, 16, 3–10.
- 4
- 4aA. Williamson, B. Conlan, W. Hillier, T. Wydrzynski, Photosynth. Res. 2011, 107, 71–86;
- 4bJ. Raymond, R. E. Blankenship, Coord. Chem. Rev. 2008, 252, 377–383.
- 5P. Du, R. Eisenberg, Energy Environ. Sci. 2012, 5, 6012–6021.
- 6
- 6aP. E. M. Siegbahn, Acc. Chem. Res. 2009, 42, 1871–1880;
- 6bS. Yamanaka, H. Isobe, K. Kanda, T. Saito, Y. Umena, K. Kawakami, J.-R. Shen, N. Kamiya, M. Okumura, H. Nakamura, K. Yamaguchi, Chem. Phys. Lett. 2011, 511, 138–145.
- 7S. Signorella, C. Hureau, Coord. Chem. Rev. 2012, 256, 1229–1245.
- 8X. Liu, F. Wang, Coord. Chem. Rev. 2012, 256, 1115–1136.
- 9For a critical evaluation of water oxidation catalysis by homogenous Mn complexes, see V. Artero, M. Fontecave, Chem. Soc. Rev. 2013, 42, 2338–2356.
- 10See the Supporting Information (Tables S1–S3) for a summary of previous reports of water oxidation by homogeneous manganese complexes as well as a comparison with other electrocatalysts based on 3d transition metals (Table S4).
- 11
- 11aY. Shimazaki, T. Nagano, H. Takesue, B.-H. Ye, F. Tani, Y. Naruta, Angew. Chem. 2004, 116, 100–102;
10.1002/ange.200352564 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 98–100;
- 11bY. Gao, T. Åkermark, J. Liu, L. Sun, B. Åkermark, J. Am. Chem. Soc. 2009, 131, 8726–8727.
- 12Recent reviews:
- 12aC. W. Cady, R. H. Crabtree, G. W. Brudvig, Coord. Chem. Rev. 2008, 252, 444–455;
- 12bM. Wiechen, H.-M. Berends, P. Kurz, Dalton Trans. 2012, 41, 21–31.
- 13Photochemical water oxidation by a multinuclear Mn complex: E. A. Karlsson, B.-L. Lee, T. Åkermark, E. V. Johnston, M. D. Kärkäs, J. Sun, Ö. Hansson, J.-E. Bäckvall, B. Åkermark, Angew. Chem. 2011, 123, 11919–11922;
10.1002/ange.201104355 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11715–11718.
- 14Electrocatalytic water oxidation in the presence of a multinuclear Mn complex in aqueous solution: Y. Gao, R. H. Crabtree, G. W. Brudvig, Inorg. Chem. 2012, 51, 4043–4050.
- 15
- 15aR. K. Seidler-Egdal, A. Nielsen, A. D. Bond, M. J. Bjerrumb, C. J. McKenzie, Dalton Trans. 2011, 40, 3849–3858;
- 15bK. J. Young, M. K. Takase, G. W. Brudvig, Inorg. Chem. 2013, 52, 7615–7622.
- 16
- 16aH. Chen, R. Tagore, G. Olack, J. S. Vrettos, T.-C. Weng, J. Penner-Hahn, R. H. Crabtree, G. W. Brudvig, Inorg. Chem. 2007, 46, 34–43;
- 16bM. Yoshida, S. Masaoka, J. Abe, K. Sakai, Chem. Asian J. 2010, 5, 2369–2378;
- 16cD. J. Wasylenko, C. Ganesamoorthy, M. A. Henderson, B. D. Koivisto, H. C. Osthoff, C. P. Berlinguette, J. Am. Chem. Soc. 2010, 132, 16094–16106;
- 16dD. J. Wasylenko, C. Ganesamoorthy, M. A. Henderson, C. P. Berlinguette, Inorg. Chem. 2011, 50, 3662–3672;
- 16eA. R. Parent, R. H. Crabtree, G. W. Brudvig, Chem. Soc. Rev. 2013, 42, 2247–2252;
- 16fD. G. H. Hetterscheid, J. N. Reek, Eur. J. Inorg. Chem. 2014, 742–749.
- 17Y. Naruta, M. Sasayama, T. Sasaki, Angew. Chem. 1994, 106, 1964–1965; Angew. Chem. Int. Ed. Engl. 1994, 33, 1839–1841.
- 18Substoichiometric amounts of O2 (maximum TON=0.05) were observed in the constant potential electrolysis of mononuclear Mn complexes in nonaqueous solvent, see T. Gao, J. H. Liu, M. Wang, Y. Na, B. Åkermark, L. Sun, Tetrahedron 2007, 63, 1987–1994.
- 19See for example,
- 19aC. Hureau, G. Blondin, M.-F. Charlot, C. Philouze, M. Nierlich, M. Césario, E. Anxolabéhère-Mallart, Inorg. Chem. 2005, 44, 3669–3683;
- 19bS. Groni, C. Hureau, R. Guillot, G. Blondin, G. Blain, E. Anxolabéhère-Mallart, Inorg. Chem. 2008, 47, 11783–11797.
- 20B. Lassalle-Kaiser, C. Hureau, D. A. Pantazis, Y. Pushkar, R. Guillot, V. K. Yachandra, J. Yano, F. Neese, E. Anxolabéhère-Mallart, Energy Environ. Sci. 2010, 3, 924–938.
- 21C. J. Gagliardi, A. K. Vannucci, J. J. Concepcion, Z. Chen, T. J. Meyer, Energy Environ. Sci. 2012, 5, 7704–7717.
- 22
- 22aC. M. Che, Z. Y. Li, K. Y. Wong, C. K. Poon, T. C. W. Mak, S. M. Peng, Polyhedron 1994, 13, 771–776;
- 22bS. P. Meneghetti, P. J. Lutz, J. Fischer, J. Kress, Polyhedron 2001, 20, 2705–2710;
- 22cM. Graf, G. Wolmershäuser, H. Kelm, S. Demeschko, F. Meyer, H.-J. Krüger, Angew. Chem. 2010, 122, 962–965;
10.1002/ange.200903789 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 950–953;
- 22dJ. R. Khusnutdinova, J. Luo, N. P. Rath, L. M. Mirica, Inorg. Chem. 2013, 52, 3920–3932.
- 23The complexes have also been crystallographically characterized as the acetonitrile solvate.
- 24B. Albela, C. Carina, S. Policar, S. Poussereau, J. Cano, J. Guilheim, L. Tchertanov, G. Blondin, M. Delroisse, J.-J. Girerd, Inorg. Chem. 2005, 44, 6959–6966.
- 25W.-T. Lee, S. Xu, D. A. Dickie, J. M. Smith, Eur. J. Inorg. Chem. 2013, 3867–3873.
- 26See the Supporting Information for more details.
- 27K. Ichinose, Y. Kimikado, T. Yoshida, Electrochemistry 2011, 79, 146–155.
- 28See for example,
- 28aJ. J. Concepcion, J. H. Jurss, M. K. Brennaman, P. G. Hoertz, A. O. T. Patrocinio, N. Y. M. Iha, J. L. Templeton, T. J. Meyer, Acc. Chem. Res. 2009, 42, 1954–1965;
- 28bS. M. Barnett, K. I. Goldberg, J. M. Mayer, Nat. Chem. 2012, 4, 498–502;
- 28cM.-T. Zhang, Z. Chen, P. Kang, T. J. Meyer, J. Am. Chem. Soc. 2013, 135, 2048–2051.
- 29C. Costentin, S. Drouet, M. Robert, J.-M. Savéant, J. Am. Chem. Soc. 2012, 134, 11235–11242.
- 30
- 30aN. D. Schley, J. D. Blakemore, N. K. Subbaiyan, C. D. Incarvito, F. D′Souza, R. H. Crabtree, G. W. Brudvig, J. Am. Chem. Soc. 2011, 133, 10473–10481;
- 30bJ. J. Stracke, R. G. Finke, J. Am. Chem. Soc. 2011, 133, 14872–14875;
- 30cB. Limburg, E. Bouwman, S. Bonnet, Coord. Chem. Rev. 2012, 256, 1451–1467.
- 31The detection limit for the DLS experiment is 40 nm, whereas the sensitivity is 0.1 ppm.
- 32
- 32aJ. J. Concepcion, J. W. Jurss, J. L. Templeton, T. J. Meyer, J. Am. Chem. Soc. 2008, 130, 16462–16463;
- 32bD. J. Wasylenko, C. Ganesamoorthy, J. Borau-Garcia, C. P. Berlinguette, Chem. Commun. 2011, 47, 4249–4251;
- 32cD. J. Wasylenko, R. D. Palmer, C. P. Berlinguette, Chem. Commun. 2013, 49, 218–227.