An Approach to Benzophosphole Oxides through Silver- or Manganese-Mediated Dehydrogenative Annulation Involving CC and CP Bond Formation†
Yuto Unoh
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Search for more papers by this authorDr. Koji Hirano
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Search for more papers by this authorCorresponding Author
Prof. Dr. Tetsuya Satoh
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
JST, ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012 (Japan)
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm===Search for more papers by this authorCorresponding Author
Prof. Dr. Masahiro Miura
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm===Search for more papers by this authorYuto Unoh
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Search for more papers by this authorDr. Koji Hirano
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Search for more papers by this authorCorresponding Author
Prof. Dr. Tetsuya Satoh
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
JST, ACT-C, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012 (Japan)
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm===Search for more papers by this authorCorresponding Author
Prof. Dr. Masahiro Miura
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan) http://www.chem.eng.osaka-u.ac.jp/∼miura-lab/index-Eng.htm===Search for more papers by this authorThis work was partly supported by Grants-in-Aid from MEXT, JSPS, and JST (Japan).
Graphical Abstract
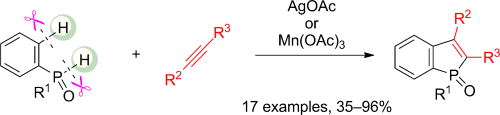
Benzophosphole construction was achieved through the AgI-mediated dehydrogenative annulation of phenylphosphine oxides with internal alkynes in a process involving CC and CP bond formation. A wide range of asymmetrical phenylacetylenes could be employed and the reactions proceeded with perfect regioselectivity. Moreover, the annulation could be conducted even at room temperature when a MnIII promoter was used in place of AgI.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307211_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Fukazawa, E. Yamaguchi, E. Ito, H. Yamada, J. Wang, S. Irle, S. Yamaguchi, Organometallics 2011, 30, 3870;
- 1bA. Fukazawa, T. Murai, L. Li, Y. Chen, S. Yamaguchi, C. R. Chim. 2010, 13, 1082;
- 1cH. Tsuji, K. Sato, Y. Sato, E. Nakamura, Chem. Asian J. 2010, 5, 1294;
- 1dA. Fukazawa, Y. Ichihashi, Y. Kosaka, S. Yamaguchi, Chem. Asian J. 2009, 4, 1729;
- 1eH. Tsuji, K. Sato, Y. Sato, E. Nakamura, J. Mater. Chem. 2009, 19, 3364;
- 1fA. Fukazawa, H. Yamada, S. Yamaguchi, Angew. Chem. 2008, 120, 5664;
10.1002/ange.200801834 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5582;
- 1gH. Tsuji, K. Sato, L. Ilies, Y. Itoh, Y. Sato, E. Nakamura, Org. Lett. 2008, 10, 2263;
- 1hA. Fukazawa, M. Hara, T. Okamoto, E. C. Son, C. Xu, K. Tamao, S. Yamaguchi, Org. Lett. 2008, 10, 913.
- 2
- 2aT. Sanji, K. Shiraishi, T. Kashiwabara, M. Tanaka, Org. Lett. 2008, 10, 2689;
- 2bJ. G. Cordaro, D. Stein, H. Grützmacher, J. Am. Chem. Soc. 2006, 128, 14962;
- 2cG. Märkl, G. Y. Jin, K. P. Berr, Tetrahedron Lett. 1993, 34, 3103;
- 2dT. Butters, W. Winter, Chem. Ber. 1984, 117, 990;
- 2eT. M. Balthazor, J. Org. Chem. 1980, 45, 2519;
- 2fW. Winter, Tetrahedron Lett. 1975, 16, 3913;
10.1016/S0040-4039(00)91252-8 Google Scholar
- 2gM. D. Rausch, L. P. Klemann, J. Am. Chem. Soc. 1967, 89, 5732. For relevant synthesis of dibenzophosphole oxides, see:
- 2hY. Kuninobu, T. Yoshida, K. Takai, J. Org. Chem. 2011, 76, 7370;
- 2iO. Berger, C. Petit, E. L. Deal, J.-L. Montchamp, Adv. Synth. Catal. 2013, 355, 1361.
- 3For earlier examples, see:
- 3aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407;
- 3bL. Li, W. W. Brennessel, W. D. Jones, J. Am. Chem. Soc. 2008, 130, 12414;
- 3cD. R. Stuart, M. Bertrand-Laperle, K. M. N. Burgess, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 16474;
- 3dD. A. Colby, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2008, 130, 3645;
- 3eS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 3fT. K. Hyster, T. Rovis, J. Am. Chem. Soc. 2010, 132, 10565;
- 3gG. Song, D. Chen, C.-L. Pan, R. H. Crabtree, X. Li, J. Org. Chem. 2010, 75, 7487.
- 4Selected reviews for transition-metal-catalyzed C–H functionalization:
- 4aD. A. Colby, A. S. Tsai, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2012, 45, 814;
- 4bK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788;
- 4cE. A. Mitchell, A. Peschiulli, N. Lefevre, L. Meerpoel, B. U. W. Maes, Chem. Eur. J. 2012, 18, 10092;
- 4dG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 4eF. W. Patureau, J. Wencel-Delord, F. Glorius, Aldrichimica Acta 2012, 45, 31;
- 4fS. H. Cho, J. Y. Kim, J. Kwak, S. Chang, Chem. Soc. Rev. 2011, 40, 5068;
- 4gJ. Wencel-Delord, T. Droge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740;
- 4hY. Kuninobu, K. Takai, Chem. Rev. 2011, 111, 1938;
- 4iC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780;
- 4jL. Ackermann, Chem. Rev. 2011, 111, 1315;
- 4kJ. Le Bras, J. Muzart, Chem. Rev. 2011, 111, 1170;
- 4lT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 4mD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 4nC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Commun. 2010, 46, 677;
- 4oT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212;
- 4pT. Satoh, M. Miura, Synthesis 2010, 3395;
- 4qB. Karimi, H. Behzadnia, D. Elhamifar, P. F. Akhavan, F. K. Esfahani, A. Zamani, Synthesis 2010, 1399;
- 4rX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 4sO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 4tSee Ref. [4s];
- 4uC.-J. Li, Acc. Chem. Res. 2009, 42, 335;
- 4vF. Kakiuchi, T. Kochi, Synthesis 2008, 3013;
- 4wY. J. Park, J.-W. Park, C.-H. Jun, Acc. Chem. Res. 2008, 41, 222;
- 4xI. V. Seregin, V. Gevorgyan, Chem. Soc. Rev. 2007, 36, 1173;
- 4yE. M. Beccalli, G. Broggini, M. Martinelli, S. Sottocornola, Chem. Rev. 2007, 107, 5318;
- 4zD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174;
- 4aaK. Godula, D. Sames, Science 2006, 312, 67.
- 5T. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141.
- 6For six-membered ring construction by annulation of diarylphosphinic acids under rhodium catalysis, see: Y. Unoh, Y. Hashimoto, D. Takeda, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2013, 15, 3258.
- 7Recently, related oxidative coupling reactions involving P-OH directed CH bond cleavage have been reported:
- 7aJ. Seo, Y. Park, I. Jeon, T. Ryu, S. Park, P. H. Lee, Org. Lett. 2013, 15, 3358;
- 7bX. Meng, S. Kim, Org. Lett. 2013, 15, 1910;
- 7cL. Y. Chan, S. Kim, T. Ryu, P. H. Lee, Chem. Commun. 2013, 49, 4682.
- 8
- 8aY.-M. Li, M. Sun, H.-L. Wang, Q.-P. Tian, S.-D. Yang, Angew. Chem. 2013, 125, 4064; Angew. Chem. Int. Ed. 2013, 52, 3972;
- 8bX. Mao, X. Ma, S. Zhang, H. Hu, C. Zhu, Y. Cheng, Eur. J. Org. Chem. 2013, 4245;
- 8cC.-B. Xiang, Y.-J. Bian, X.-R. Mao, Z.-Z. Huang, J. Org. Chem. 2012, 77, 7706;
- 8dF. Effenberger, H. Kottmann, Tetrahedron 1985, 41, 4171. For related Ag-mediated C–H/C–H dehydrogenative coupling, see:
- 8eC. He, S. Guo, J. Ke, J. Hao, H. Xu, H. Chen, A. Lei, J. Am. Chem. Soc. 2012, 134, 5766.
- 9U. Wille, Chem. Rev. 2013, 113, 813.
- 10
- 10aJ. Zhou, G.-L. Zhang, J.-P. Zou, W. Zhang, Eur. J. Org. Chem. 2011, 3412;
- 10bF. Beaufils, F. Dénès, P. Renaud, Angew. Chem. 2005, 117, 5407;
10.1002/ange.200501309 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5273;
- 10cA. Sato, H. Yorimitsu, K. Oshima, Angew. Chem. 2005, 117, 1722; Angew. Chem. Int. Ed. 2005, 44, 1694;
- 10dA. Kondoh, H. Yorimitsu, K. Oshima, Chem. Asian J. 2010, 5, 398. For related addition of S- or C-radical/cyclization, see:
- 10eK. Liu, F. Jia, H. Xi, Y. Li, X. Zheng, Q. Guo, B. Shen, Z. Li, Org. Lett. 2013, 15, 2026;
- 10fD. P. Hari, T. Hering, B. König, Org. Lett. 2012, 14, 5334;
- 10gM. Tobisu, K. Koh, T. Furukawa, N. Chatani, Angew. Chem. 2012, 124, 11525;
10.1002/ange.201206115 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11363;
- 10hL. Benati, G. Calestani, R. Leardini, M. Minozzi, D. Nanni, P. Spagnolo, S. Strazzari, G. Zanardi, J. Org. Chem. 2003, 68, 3454.
- 11
- 11aX.-Q. Pan, J.-P. Zou, G.-L. Zhang, W. Zhang, Chem. Commun. 2010, 46, 1721;
- 11bO. Tayama, A. Nakano, T. Iwahama, S. Sakaguchi, Y. Ishii, J. Org. Chem. 2004, 69, 5494;
- 11cT. Nagayama, A. Nakano, S. Sakaguchi, Y. Ishii, Org. Lett. 2006, 8, 407. For a review, see:
- 11dB. B. Snider, Chem. Rev. 1996, 96, 339.
- 12Compound 3 aq seems to be formed as a mixture of diastereomers. However, the ratio could not be determined owing to overlap in the NMR chemical shifts.
- 13
- 13aB. S. Bhakuni, A. Kumar, S. J. Balkrishna, J. A. Sheikh, S. Konar, S. Kumar, Org. Lett. 2012, 14, 2838;
- 13bD. S. Roman, Y. Takahashi, A. B. Charette, Org. Lett. 2011, 13, 3242.
- 14It is also possible that the 4-exo-trig cyclization is promoted by the methyl group as a result of its stabilization of the corresponding spirocyclohexadienyl radical through hyperconjugation.