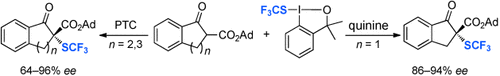Enantioselective Electrophilic Trifluoromethylthiolation of β-Ketoesters: A Case of Reactivity and Selectivity Bias for Organocatalysis†
Xueqiang Wang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
These authors contributed equally to this work.
Search for more papers by this authorTao Yang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiaolin Cheng
Center for Molecular Biophysics, Oak Ridge National Laboratory, 1 Bethel Valley Rd, Oak Ridge, TN 37831 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Qilong Shen
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)===Search for more papers by this authorXueqiang Wang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
These authors contributed equally to this work.
Search for more papers by this authorTao Yang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiaolin Cheng
Center for Molecular Biophysics, Oak Ridge National Laboratory, 1 Bethel Valley Rd, Oak Ridge, TN 37831 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Qilong Shen
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (P.R. China)===Search for more papers by this authorThe authors gratefully acknowledge financial support from the National Basic Research Program of China (2012CB821600), the Key Program of Natural Science Foundation of China (21032006), the National Natural Science Foundation of China (21172245/21172244/B020304), the Shanghai Pujiang Program (11J1412200), and SIOC.
Graphical Abstract
A chiral Lewis base or a phase-transfer catalyst (PTC) can mediate the highly enantioselective trifluoromethylthiolation of β-ketoesters with the previously developed SCF3 reagent (see scheme). Reactions of indanone-derived β-ketoesters occurred with high yield and excellent enantioselectivity with quinine as catalyst. Reactions of tetralone- or 1-benzosuberone-derived β-ketoesters occurred with moderate to good enantioselectivity with a quinine-derived PTC.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305075_sm_miscellaneous_information.pdf8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Becker, Inventory of Industrial Fluoro-Biochemicals, Eyrolles, Paris, 1996;
- 1bV. N. Boiko, Beilstein J. Org. Chem. 2010, 6, 880.
- 2
- 2aA. Leo, C. Hansch, D. Elkins, Chem. Rev. 1971, 71, 525;
- 2bC. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165;
- 2cR. Filler, Biomedical Aspects of Fluorine Chemistry, Kodansha, Tokyo, 1982;
- 2dL. M. Yagupol’skii, A. Y. Ilchenko, N. V. Kondratenko, Russ. Chem. Rev. 1974, 43, 32;
10.1070/RC1974v043n01ABEH001787 Google Scholar
- 2eF. Leroux, P. Jeschke, M. Schlosser, Chem. Rev. 2005, 105, 827;
- 2fB. Manteau, S. Pazenok, J.-P. Vors, F. R. Leroux, J. Fluorine Chem. 2010, 131, 140.
- 3A. Tlili, T. Billard, Angew. Chem. 2013, 125, 6952–6954;
10.1002/ange.201301438 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 6818–6819.
- 4
- 4aG. Teverovskiy, D. S. Surry, S. L. Buchwald, Angew. Chem. 2011, 123, 7450–7452;
10.1002/ange.201102543 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7312–7314;
- 4bC.-P. Zhang, D. A. Vicic, J. Am. Chem. Soc. 2012, 134, 183;
- 4cC.-P. Zhang, D. A. Vicic, Chem. Asian J. 2012, 7, 1756;
- 4dC. Chen, L.-L. Chu, F.-L. Qing, J. Am. Chem. Soc. 2012, 134, 12454;
- 4eC. Chen, Y. Xie, L.-L. Chu, R.-W. Wang, X.-G. Zhang, F.-L. Qing, Angew. Chem. 2012, 124, 2542–2545; Angew. Chem. Int. Ed. 2012, 51, 2492–2495;
- 4fL. D. Tran, I. Popov, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 18237;
- 4gZ. Weng, W. He, C. Chen, R. Lee, D. Tan, Z. Lai, D. Kong, Y. Yuan, K.-W. Huang, Angew. Chem. 2013, 125, 1588–1592; Angew. Chem. Int. Ed. 2013, 52, 1548–1552;
- 4hF. Baert, J. Colomb, T. Billard, Angew. Chem. 2012, 124, 10528–10531;
10.1002/ange.201205156 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10382–10385;
- 4iH. Yasui, T. Yamamoto, E. Tokunaga, N. Shibata, J. Fluorine Chem. 2011, 132, 186;
- 4jY.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro, N. Shibata, J. Am. Chem. Soc. 2013, 135, 8782.
- 5
- 5aJ.-A. Ma, D. Cahard, Chem. Rev. 2004, 104, 6119;
- 5bJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1;
- 5cJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2011, 111, 455;
- 5dN. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633;
- 5eP. V. Ramachandran in Asymmetric Fluoroorganic Chemistry: Synthesis, Applications, and Future Directions, American Chemical Society Symposium Series 746, American Chemical Society, Washington DC, 2000.
- 6Selected examples of asymmetric trifluoromethylation methods:
- 6aV. Bizet, X. Pannecoucke, J. Renaud, D. Cahard, Angew. Chem. 2012, 124, 6573–6576;
10.1002/ange.201200827 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6467–6470;
- 6bD. A. Nagib, M. E. Scott, D. W. C. MacMillan, J. Am. Chem. Soc. 2009, 131, 10875;
- 6cA. E. Allen, D. W. C. MacMillan, J. Am. Chem. Soc. 2010, 132, 4986;
- 6dQ.-H. Deng, H. Wadepohl, L. H. Gade, J. Am. Chem. Soc. 2012, 134, 10769;
- 6eA. T. Herrmann, L. L. Smith, A. Zakarian, J. Am. Chem. Soc. 2012, 134, 6976;
- 6fH. Kawai, A. Kusuda, S. Nakamura, M. Shiro, N. Shibata, Angew. Chem. 2009, 121, 6442–6445; Angew. Chem. Int. Ed. 2009, 48, 6324–6327;
- 6gS. Noritake, N. Shibata, Y. Nomura, Y. Huang, A. Matsnev, S. Nakamura, T. Toru, D. Cahard, Org. Biomol. Chem. 2009, 7, 3599;
- 6hT. Umemoto, K. Adachi, J. Org. Chem. 1994, 59, 5692;
- 6iJ.-R. Gao, H. Wu, B. Xiang, W.-B. Yu, L. Han, Y.-X. Jia, J. Am. Chem. Soc. 2013, 135, 2983;
- 6jY. Huang, E. Tokunaga, S. Suzuki, M. Shiro, N. Shibata, Org. Lett. 2010, 12, 1136;
- 6kG. Blay, I. Fernandez, M. C. Munoz, J. R. Pedro, C. Vila, Chem. Eur. J. 2010, 16, 9117;
- 6lJ.-H. Lin, J.-C. Xiao, Eur. J. Org. Chem. 2011, 4536;
- 6mW. Wang, X. Lian, D. Chen, X. Liu, L. Lin, X. Feng, Chem. Commun. 2011, 47, 7821;
- 6nL. Wen, Q. Shen, X. Wan, L. Lu, J. Org. Chem. 2011, 76, 2282;
- 6oK. Shibatomi, A. Narayama, Y. Abe, S. Iwasa, Chem. Commun. 2012, 48, 7380.
- 7X. Shao, X. Wang, T. Yang, L. Lu, Q. Shen, Angew. Chem. 2013, 125, 3541–3544; Angew. Chem. Int. Ed. 2013, 52, 3457–3460.
- 8Review:
- 8aH.-M. Li, Y.-G. Chen, L. Deng in Privileged Chiral Ligands and Catalysts (Eds.: ), Wiley-VCH, Weinheim, 2011;
10.1002/9783527635207.ch10 Google Scholar
- 8bS.-K. Tian, Y.-G. Chen, J.-F. Hang, L. Tang, P. Mcdaid, L. Deng, Acc. Chem. Res. 2004, 37, 621;
- 8cS. France, A. Weatherwax, A. E. Taggi, T. Lectka, Acc. Chem. Res. 2004, 37, 592;
- 8dP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248–5286;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138–5175;
- 8eK. Kacprzak, J. Gawroñski, Synthesis 2001, 961.
- 9Selected examples of asymmetric reactions in fluorine chemistry catalyzed by cinchona alkaloids and their derivatives:
- 9aB. Mohar, J. Baudoux, J.-C. Plaquevent, D. Cahard, Angew. Chem. 2001, 113, 4339–4341;
10.1002/1521-3757(20011119)113:22<4339::AID-ANGE4339>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4214–4216;10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 9bN. Shibata, E. Suzuki, Y. Takeuchi, J. Am. Chem. Soc. 2000, 122, 10728;
- 9cN. Shibata, E. Suzuki, T. Asahi, M. Shiro, J. Am. Chem. Soc. 2001, 123, 7001;
- 9dT. Ishimaru, N. Shibata, T. Horikawa, N. Yasuda, S. Nakamura, T. Toru, M. Shiro, Angew. Chem. 2008, 120, 4225–4229;
10.1002/ange.200800717 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4157–4161;
- 9eL. Zoute, C. Audouard, J.-C. Plaquevent, D. Cahard, Org. Biomol. Chem. 2003, 1, 1833;
- 9fN. Shibata, T. Ishimaru, M. Nakamura, T. Toru, Synlett 2004, 2509;
- 9gS. Arai, M. Oku, T. Ishida, T. Shioiri, Tetrahedron Lett. 1999, 40, 6785;
- 9hJ. Baudoux, D. Cahard, Organic Reactions, Hoboken, NJ, 2007, 69, 347;
- 9iS. Ogawa, N. Shibata, J. Inagaki, S. Nakamura, T. Toru, M. Shiro, Angew. Chem. 2007, 119, 8820–8823;
10.1002/ange.200703317 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8666–8669;
- 9jB. Török, M. Abid, G. London, J. Esquibel, M. Török, S. C. Mhadgut, P. Yan, G. K. S. Prakash, Angew. Chem. 2005, 117, 3146–3149;
10.1002/ange.200462877 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3086–3089;
- 9kJ.-L. Zhao, L. Liu, C.-L. Gu, D. Wang, Y.-J. Chen, Tetrahedron Lett. 2008, 49, 1476;
- 9lM. Bandini, R. Sinisi, A. Umani-Ronchi, Chem. Commun. 2008, 4360;
- 9mT. Furukawa, T. Nishimine, E. Tokunaga, K. Hasegawa, M. Shir, N. Shibata, Org. Lett. 2011, 13, 3972;
- 9nY. Li, F. Lian, Q. Li, Y.-C. Xu, Q.-R. Wang, L. Jang, Org. Lett. 2011, 13, 6082.
- 10CCDC 952517 (4 e) and 952518 (5 f) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11This Communication is published back-to-back with the following study: T. Bootwicha, X. Liu, R. Pluta, I. Atodiresei, M. Rueping, Angew. Chem. 2013, 125, 13093–13097; Angew. Chem. Int. Ed. 2013, 52, 12856–12859.