A Twin-Axial Hetero[7]rotaxane†
Zhi-Jun Zhang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorProf. Dr. Heng-Yi Zhang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorHui Wang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yu Liu
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)Search for more papers by this authorZhi-Jun Zhang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorProf. Dr. Heng-Yi Zhang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorHui Wang
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yu Liu
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Department of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 (P.R. China)Search for more papers by this authorWe thank the 973 Program (2011CB932500), and the NNSFC (Nos. 20932004 and 20972077) for financial support.
Graphical Abstract
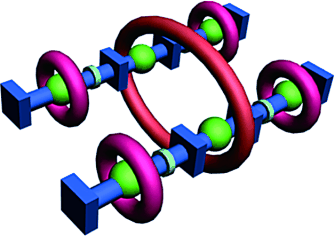
Two in one: Two pseudorotaxanes can be combined to form a twin-axial hetero[7]rotaxane (see picture) by using the copper-catalyzed alkyne–azide “click” reaction. The synthetic route, in which twin-axial and single-axial rotaxanes are formed, combines self-assembly and the formation of covalent bonds to ensure the correct positioning of the two types of rings in the final product.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105375_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Molecular Catenanes, Rotaxanes and Knots (Eds.: ), Wiley-VCH, Weinheim, 1999;
- 1bV. Balzani, A. Credi, M. Venturi, Molecular Devices and Machines—Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, 2008;
10.1002/9783527621682 Google Scholar
- 1cV. Balzani, A. Credi, F. M. Raymo, J. F. Stoddart, Angew. Chem. 2000, 112, 3484–3530;
10.1002/1521-3757(20001002)112:19<3484::AID-ANGE3484>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3348–3391;10.1002/1521-3773(20001002)39:19<3348::AID-ANIE3348>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 1dE. R. Kay, D. A. Leigh, F. Zerbetto, Angew. Chem. 2007, 119, 72–196; Angew. Chem. Int. Ed. 2007, 46, 72–191;
- 1eA. Harada, A. Hashidzume, H. Yamaguchi, Y. Takashima, Chem. Rev. 2009, 109, 5974–6023;
- 1fX. Ma, H. Tian, Chem. Soc. Rev. 2010, 39, 70–80;
- 1gD.-H. Qu, H. Tian, Chem. Sci. 2011, 2, 1011–1015.
- 2
- 2aF. Arico, J. D. Badjic, S. J. Cantrill, A. H. Flood, K. C. F. Leung, Y. Liu, J. F. Stoddart, Top. Curr. Chem. 2005, 249, 203–259;
- 2bC. Yang, T. Mori, Y. Origane, Y. H. Ko, N. Selvapalam, K. Kim, Y. Inoue, J. Am. Chem. Soc. 2008, 130, 8574–8575; for the “threading-followed-by-shrinking” protocol, see:
- 2cI. Yoon, M. Narita, T. Shimizu, M. Asakawa, J. Am. Chem. Soc. 2004, 126, 16740–16741;
- 2dS. Y. Hsueh, J. L. Ko, C. C. Lai, Y. H. Liu, S. M. Peng, S. H. Chiu, Angew. Chem. 2011, 123, 6773–6776;
10.1002/ange.201101524 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6643–6646; for the “threading-followed-by-swelling” protocol, see:
- 2eC. W. Chiu, C. C. Lai, S. H. Chiu, J. Am. Chem. Soc. 2007, 129, 3500–3501.
- 3For examples of polyrotaxanes, see:
- 3aJ. Wu, K. C. F. Leung, J. F. Stoddart, Proc. Natl. Acad. Sci. USA 2007, 104, 17266–17271;
- 3bW. Y. Zhang, W. R. Dichtel, A. Z. Stieg, D. Benitez, J. K. Gimzewski, J. R. Heath, J. F. Stoddart, Proc. Natl. Acad. Sci. USA 2008, 105, 6514–6519;
- 3cD. Tuncel, J. H. G. Steinke, Macromolecules 2004, 37, 288–302;
- 3dY. G. Lee, Y. Koyama, M. Yonekawa, T. Takata, Macromolecules 2010, 43, 4070–4080;
- 3eT. Bilig, T. Oku, Y. Furusho, Y. Koyama, S. Asai, T. Takata, Macromolecules 2008, 41, 8496–8503;
- 3fY. Liu, P. Liang, Y. Chen, Y. M. Zhang, J. Y. Zheng, H. Yue, Macromolecules 2005, 38, 9095–9099;
- 3gM. E. Belowich, C. Valente, J. F. Stoddart, Angew. Chem. 2010, 122, 7366–7370;
10.1002/ange.201004304 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7208–7212.
- 4For examples of oligorotaxanes, see:
- 4aJ. Yin, S. Dasgupta, J. Wu, Org. Lett. 2010, 12, 1712–1715;
- 4bQ. Jiang, H. Y. Zhang, M. Han, Z. J. Ding, Y. Liu, Org. Lett. 2010, 12, 1728–1731;
- 4cS. J. Loeb, D. A. Tramontozzi, Org. Biomol. Chem. 2005, 3, 1393–1401;
- 4dK. C. Leung, F. Arico, S. J. Cantrill, J. F. Stoddart, J. Am. Chem. Soc. 2005, 127, 5808–5810;
- 4eA. M. Fuller, D. A. Leigh, P. J. Lusby, J. Am. Chem. Soc. 2010, 132, 4954–4959;
- 4fW. R. Dichtel, O. S. Miljanic, J. M. Spruell, J. R. Heath, J. F. Stoddart, J. Am. Chem. Soc. 2006, 128, 10388–10390;
- 4gL. Jiang, J. Okano, A. Orita, J. Otera, Angew. Chem. 2004, 116, 2173–2176; Angew. Chem. Int. Ed. 2004, 43, 2121–2124;
- 4hA. M. Fuller, D. A. Leigh, P. J. Lusby, Angew. Chem. 2007, 119, 5103–5107;
10.1002/ange.200700933 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5015–5019;
- 4iH. W. Daniell, E. J. Klotz, B. Odell, T. D. Claridge, H. L. Anderson, Angew. Chem. 2007, 119, 6969–6972;
10.1002/ange.200702349 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6845–6848;
- 4jS. Y. Hsueh, K. W. Cheng, C. C. Lai, S. H. Chiu, Angew. Chem. 2008, 120, 4508–4511;
10.1002/ange.200800530 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4436–4439;
- 4kY. Jiang, J. B. Guo, C. F. Chen, Org. Lett. 2010, 12, 4248–4251.
- 5
- 5aW. Jiang, H. D. F. Winkler, C. A. Schalley, J. Am. Chem. Soc. 2008, 130, 13852–13853;
- 5bJ. Li, C. Yang, X. P. Ni, Polymer 2009, 50, 4496–4504. For examples of heterowheel pseudorotaxanes see:
- 5cW. Jiang, C. A. Schalley, Proc. Natl. Acad. Sci. USA 2009, 106, 10425–10429;
- 5dT. Ooya, D. Inoue, H. S. Choi, Y. Kobayashi, S. Loethen, D. H. Thompson, Y. H. Ko, K. Kim, N. Yui, Org. Lett. 2006, 8, 3159–3162;
- 5eE. Masson, X. Lu, X. Ling, D. L. Patchell, Org. Lett. 2009, 11, 3798–3801;
- 5fZ. J. Ding, H. Y. Zhang, L. H. Wang, F. Ding, Y. Liu, Org. Lett. 2011, 13, 856–859;
- 5gJ. Yin, C. Chi, J. Wu, Org. Biomol. Chem. 2010, 8, 2594–2599;
- 5hW. Jiang, A. Schafer, P. C. Mohr, C. A. Schalley, J. Am. Chem. Soc. 2010, 132, 2309–2320;
- 5iW. Jiang, Q. Wang, I. Linder, F. Klautzsch, C. A. Schalley, Chem. Eur. J. 2011, 17, 2344–2348;
- 5jG. Celtek, M. Artar, O. A. Scherman, D. Tuncel, Chem. Eur. J. 2009, 15, 10360–10363;
- 5kX. Ma, Q. C. Wang, D. H. Qu, Y. Xu, F. Y. Ji, H. Tian, Adv. Funct. Mater. 2007, 17, 829–837.
- 6
- 6aH. M. Cheng, D. A. Leigh, F. Maffei, P. R. McGonigal, A. M. Z. Slawin, J. Wu, J. Am. Chem. Soc. 2011, 133, 12298–12303;
- 6bC. F. Lee, D. A. Leigh, R. G. Pritchard, D. Schultz, S. J. Teat, G. A. Timco, R. E. Winpenny, Nature 2009, 458, 314–318;
- 6cD. Ackermann, T. L. Schmidt, J. S. Hannam, C. S. Purohit, A. Heckel, M. Famulok, Nat. Nanotechnol. 2010, 5, 436–442;
- 6dA. I. Prikhod’ko, J. P. Sauvage, J. Am. Chem. Soc. 2009, 131, 6794–6807;
- 6eA. I. Prikhod’ko, F. Durola, J. P. Sauvage, J. Am. Chem. Soc. 2008, 130, 448–449;
- 6fE. J. Klotz, T. D. Claridge, H. L. Anderson, J. Am. Chem. Soc. 2006, 128, 15374–15375;
- 6gC. Yang, Y. H. Ko, N. Selvapalam, Y. Origane, T. Mori, T. Wada, K. Kim, Y. Inoue, Org. Lett. 2007, 9, 4789–4792.
- 7
- 7aJ. D. Badjic, V. Balzani, A. Credi, S. Silvi, J. F. Stoddart, Science 2004, 303, 1845–1849;
- 7bX. Z. Zhu, C. F. Chen, J. Am. Chem. Soc. 2005, 127, 13158–13159;
- 7cD. A. Leigh, V. Serreli, C. F. Lee, E. R. Kay, Nature 2007, 445, 523–527.
- 8
- 8aP. R. Ashton, P. J. Campbell, P. T. Glink, D. Philp, N. Spencer, J. F. Stoddart, E. J. T. Chrystal, S. Menzer, D. J. Williams, P. A. Tasker, Angew. Chem. 1995, 107, 1997–2001;
10.1002/ange.19951071711 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1865–1869;
- 8bP. R. Ashton, E. J. T. Chrystal, P. T. Glink, S. Menzer, C. Schiavo, J. F. Stoddart, P. A. Tasker, A. J. P. White, D. J. Williams, Chem. Eur. J. 1996, 2, 709–728;
- 8cA. G. Kolchinski, D. H. Busch, N. W. Alcock, J. Chem. Soc. Chem. Commun. 1995, 1289–1291.
- 9
- 9aP. R. Ashton, P. T. Glink, M.-V. Martínez-Díaz, J. F. Stoddart, A. J. P. White, D. J. Williams, Angew. Chem. 1996, 108, 2058–2061;
10.1002/ange.19961081709 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1930–1933;
- 9bP. R. Ashton, M. C. T. Fyfe, M.-V. Martínez-Díaz, S. Menzer, C. Schiavo, J. F. Stoddart, A. J. P. White, D. J. Williams, Chem. Eur. J. 1998, 4, 1523–1534;
10.1002/(SICI)1521-3765(19980807)4:8<1523::AID-CHEM1523>3.0.CO;2-V CAS Web of Science® Google Scholar
- 9cP. R. Ashton, R. Ballardini, V. Balzani, M. C. T. Fyfe, M. T. Gandolfi, M.-V. Martínez-Díaz, M. Morosini, C. Schiavo, K. Shibata, J. F. Stoddart, A. J. P. White, D. J. Williams, Chem. Eur. J. 1998, 4, 2332–2341.
10.1002/(SICI)1521-3765(19981102)4:11<2332::AID-CHEM2332>3.0.CO;2-S CAS Web of Science® Google Scholar
- 10C. Zhang, S. Li, J. Zhang, K. Zhu, N. Li, F. Huang, Org. Lett. 2007, 9, 5553–5556.
- 11
- 11aF. Coutrot, E. Busseron, Chem. Eur. J. 2008, 14, 4784–4787;
- 11bK. D. Hänni, D. A. Leigh, Chem. Soc. Rev. 2010, 39, 1240–1251;
- 11cI. Aprahamian, O. S. Miljanic, W. R. Dichtel, K. Isoda, T. Yasuda, T. Kato, J. F. Stoddart, Bull. Chem. Soc. Jpn. 2007, 80, 1856–1869.
- 12S. H. Chiu, A. R. Pease, J. F. Stoddart, A. J. White, D. J. Williams, Angew. Chem. 2002, 114, 280–284; Angew. Chem. Int. Ed. 2002, 41, 270–274.
- 13W. Jiang, C. A. Schalley, Beilstein J. Org. Chem. 2010, DOI: .
- 14P.-L. Anelli, P. R. Ashton, R. Ballardini, V. Balzani, M. Delgado, M. T. Gandolfi, T. T. Goodnow, A. E. Kaifer, D. Philp, M. Pietraszkiewicz, L. Prodi, M. V. Reddington, A. M. Z. Slawin, N. Spencer, J. F. Stoddart, C. Vicent, D. J. Williams, J. Am. Chem. Soc. 1992, 114, 193–218.
Citing Literature
November 11, 2011
Pages 10834-10838