DNA-Programmed Glaser–Eglinton Reactions for the Synthesis of Conjugated Molecular Wires†
Jens B. Ravnsbæk
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorMikkel F. Jacobsen
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorChristian B. Rosen
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorNiels V. Voigt
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorCorresponding Author
Prof. Kurt V. Gothelf
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)Search for more papers by this authorJens B. Ravnsbæk
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorMikkel F. Jacobsen
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorChristian B. Rosen
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorNiels V. Voigt
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Search for more papers by this authorCorresponding Author
Prof. Kurt V. Gothelf
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)
Danish National Research Foundation: Center for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C (Denmark)Search for more papers by this authorThe work was supported by the Danish National Research Foundation.
Graphical Abstract
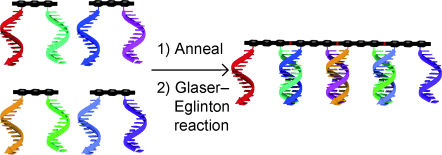
Wire self-assembly: Short oligo(phenylene ethynylene) modules (black structures, see picture) are assembled by attached DNA strands, which also direct the formation of 1,3-diyne linkages between the modules by the Cu-mediated Glaser–Eglinton reaction to selectively form dimer, trimer, and tetramer conjugated wires of up to 8 nm in length.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105095_sm_miscellaneous_information.pdf965.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. J. Ashwell, R. Hamilton, L. R. H. High, J. Mater. Chem. 2003, 13, 1501–1503;
- 1bS. Kubatkin, A. Danilov, M. Hjort, J. Cornil, J. L. Brédas, N. Stuhr-Hansen, P. Hedegård, T. Bjørnholm, Nature 2003, 425, 698–701;
- 1cJ. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johnston-Halperin, E. Delonno, Y. Luo, B. A. Sheriff, K. Xu, Y. S. Shin, H. R. Tseng, J. F. Stoddart, J. R. Heath, Nature 2007, 445, 414–417;
- 1dD. W. Steuerman, H. R. Tseng, A. J. Peters, A. H. Flood, J. O. Jeppesen, K. A. Nielsen, J. F. Stoddart, J. R. Heath, Angew. Chem. 2004, 116, 6648–6653;
10.1002/ange.200461723 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6486–6491;
- 1eL. A. Bumm, J. J. Arnold, M. T. Cygan, T. D. Dunbar, T. P. Burgin, L. Jones II, D. L. Allara, J. M. Tour, P. S. Weiss, Science 1996, 271, 1705–1707.
- 2
- 2aC. Joachim, J. K. Gimzewski, A. Aviram, Nature 2000, 408, 541–548;
- 2bN. Robertson, G. A. McGovan, Chem. Soc. Rev. 2003, 32, 96–103;
- 2cJ. M. Tour, Acc. Chem. Res. 2000, 33, 791–804.
- 3
- 3aK. V. Gothelf, T. H. Labean, Org. Biomol. Chem. 2005, 3, 4023–4037;
- 3bN. C. Seeman, Nature 2003, 421, 427–431;
- 3cU. Feldkamp, C. M. Niemeyer, Angew. Chem. 2006, 118, 1888–1910;
10.1002/ange.200502358 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1856–1876;
- 3dJ. Bath, A. J. Turberfield, Nat. Nanotechnol. 2007, 2, 275–284;
- 3eY. Krishnan, F. C. Simmel, Angew. Chem. 2011, 123, 3180–3215;
10.1002/ange.200907223 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3124–3156.
- 4
- 4aZ. J. Gartner, D. R. Liu, J. Am. Chem. Soc. 2001, 123, 6961–6963;
- 4bZ. J. Gartner, M. W. Kanan, D. R. Liu, Angew. Chem. 2002, 114, 1874–1878;
10.1002/1521-3757(20020517)114:10<1874::AID-ANGE1874>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1796–1800;10.1002/1521-3773(20020517)41:10<1796::AID-ANIE1796>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 4cZ. J. Gartner, R. Grubina, C. T. Calderone, D. R. Liu, Angew. Chem. 2003, 115, 1408–1413;
10.1002/ange.200390323 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1370–1375.
- 5
- 5aZ. J. Gartner, B. N. Tse, R. Grubina, J. B. Doyon, T. M. Snyder, D. R. Liu, Science 2004, 305, 1601–1606;
- 5bN. B. Tse, T. M. Snyder, Y. Shen, D. R. Liu, J. Am. Chem. Soc. 2008, 130, 15611–15626;
- 5cM. H. Hansen, P. Blakskjær, L. K. Petersen, T. H. Hansen, J. W. Højfeldt, K. V. Gothelf, N. V. Hansen, J. Am. Chem. Soc. 2009, 131, 1322–1327.
- 6
- 6aH. T. Maune, S. Han, R. D. Barish, M. Bockrath, W. A. Goddard III, P. W. K. Rothemund, E. Winfree, Nat. Nanotechnol. 2010, 5, 61–66;
- 6bK. V. Gothelf, A. H. Thomsen, M. Nielsen, E. Cló, R. S. Brown, J. Am. Chem. Soc. 2004, 126, 1044–1046;
- 6cM. Nielsen, A. H. Thomsen, E. Cló, F. Kirpekar, K. V. Gothelf, J. Org. Chem. 2004, 69, 2240–2250;
- 6dR. S. Brown, M. Nielsen, K. V. Gothelf, Chem. Commun. 2004, 1464–1465;
- 6eK. V. Gothelf, R. S. Brown, Chem. Eur. J. 2005, 11, 1062–1069;
- 6fM. Nielsen, V. Dauksaite, J. Kjems, K. V. Gothelf, Bioconjugate Chem. 2005, 16, 981–985;
- 6gP. Blakskjær, K. V. Gothelf, Org. Biomol. Chem. 2006, 4, 3442–3447;
- 6hA. P. Eskelinen, A. Kuzyk, T. K. Kaltiaisenaho, M. Y. Timmermans, A. G. Nasibulin, E. I. Kauppinen, P. Törmä, Small 2011, 7, 746–750.
- 7J. L. Czlapinski, T. L. Sheppard, J. Am. Chem. Soc. 2001, 123, 8618–8619.
- 8
- 8aG. Sedghi, K. Sawada, L. J. Esdaile, M. Hoffmann, H. L. Anderson, D. Bethell, W. Haiss, S. J. Higgins, R. J. Nichols, J. Am. Chem. Soc. 2008, 130, 8582–8583;
- 8bM. U. Winters, E. Dahlstedt, H. E. Blades, C. J. Wilson, M. J. Frampton, H. L. Anderson, B. Albinsson, J. Am. Chem. Soc. 2007, 129, 4291–4297;
- 8cM. Wielopolski, C. Atienza, T. Clark, D. M. Guldi, N. Martín, Chem. Eur. J. 2008, 14, 6379–6390.
- 9
- 9aC. Glaser, Ber. Dtsch. Chem. Ges. 1869, 2, 422–424;
10.1002/cber.186900201183 Google Scholar
- 9bC. Glaser, Justus Liebigs Ann. Chem. 1870, 154, 137–171;
10.1002/jlac.18701540202 Google Scholar
- 9cG. Eglinton, A. R. Galbraith, Chem. Ind. 1956, 737–738.
- 10The notation “Glaser–Eglinton” is used, since both CuI, CuII, and mixtures thereof are used in the reactions. Mechanistically, the reactions are similar, the difference being the use of catalytic CuI in Glaser reactions, which is reoxidized in the catalytic cycle by oxygen in the reaction medium.
- 11
- 11aS. Anderson, R. T. Aplin, T. D. W. Claridge, T. Goodson III, A. C. Maciel, G. Rumbles, J. F. Ryan, H. L. Anderson, J. Chem. Soc. Perkin Trans. 1 1998, 2383–2397;
- 11bJ. B. Armitage, E. R. H. Jones, M. C. Whitning, J. Chem. Soc. 1952, 2014–2018.
- 12Tris-hydroxypropyltriazolylamine (THTA), see
- 12aT. R. Chan, R. Hilgraf, K. B. Sharpless, V. V. Fokin, Org. Lett. 2004, 6, 2853–2855;
- 12bM. F. Jacobsen, J. B. Ravnsbæk, K. V. Gothelf, Org. Biomol. Chem. 2010, 8, 50–52.
- 13Bathophenanthroline disulfonic acid disodium (BPDS), see
- 13aW. G. Lewis, F. G. Magallon, V. V. Fokin, M. G. Finn, J. Am. Chem. Soc. 2004, 126, 9152–9253;
- 13bV. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim, M. G. Finn, J. Am. Chem. Soc. 2007, 129, 12696–12704.
- 14
- 14aC.-H. Chen, L. Milne, R. Landgraf, D. M. Perrin, D. S. Sigman, ChemBioChem 2001, 2, 735–740;
10.1002/1439-7633(20011001)2:10<735::AID-CBIC735>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 14bB. C. Bales, T. Kodama, Y. N. Weledji, M. Pitié, B. Meunier, M. M. Greenberg, Nucleic Acids res. 2005, 33, 5371–5379.
- 15R. Kumar, A. El-Sagheer, J. Tumpane, P. Lincoln, L. M. Wilhelmsson, T. Brown, J. Am. Chem. Soc. 2007, 129, 6859–6864.
- 16P. Siemsen, R. C. Livingston, F. Diederich, Angew. Chem. 2000, 112, 2740–2767;
10.1002/1521-3757(20000804)112:15<2740::AID-ANGE2740>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2632–2657.10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 17O. Henze, D. Lentz, A. D. Schlüter, Chem. Eur. J. 2000, 6, 2362–2367.
10.1002/1521-3765(20000703)6:13<2362::AID-CHEM2362>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 18P. W. K. Rothemund, Nature 2006, 440, 297–302.
- 19
- 19aN. V. Voigt, T. Tørring, A. Rotaru, M. F. Jacobsen, J. B. Ravnsbæk, R. Subramani, W. Mamdouh, J. Kjems, A. Mokhir, F. Besenbacher, K. V. Gothelf, Nat. Nanotechnol. 2010, 5, 200–203;
- 19bT. Tørring, N. V. Voigt, J. Nangreave, H. Yan, K. V. Gothelf, Chem. Soc. Rev. 2011, DOI: .