Selective Adsorption of CO2 from Light Gas Mixtures by Using a Structurally Dynamic Porous Coordination Polymer†
Dr. Kristi L. Kauffman
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDr. Jeffrey T. Culp
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
URS, P.O. Box 618, South Park, PA 15129 (USA)
Search for more papers by this authorDr. Andrew J. Allen
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorDr. Laura Espinal
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorDr. Winnie Wong-Ng
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorThomas D. Brown
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDr. Angela Goodman
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorMark P. Bernardo
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorRussel J. Pancoast
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDanielle Chirdon
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorCorresponding Author
Dr. Christopher Matranga
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)Search for more papers by this authorDr. Kristi L. Kauffman
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDr. Jeffrey T. Culp
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
URS, P.O. Box 618, South Park, PA 15129 (USA)
Search for more papers by this authorDr. Andrew J. Allen
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorDr. Laura Espinal
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorDr. Winnie Wong-Ng
National Institute of Standards and Technology, United States Department of Commerce, Gaithersburg, MD 20899 (USA)
Search for more papers by this authorThomas D. Brown
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDr. Angela Goodman
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorMark P. Bernardo
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorRussel J. Pancoast
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorDanielle Chirdon
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
Search for more papers by this authorCorresponding Author
Dr. Christopher Matranga
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)
National Energy Technology Laboratory, United States Department of Energy, P.O. Box 10940, Pittsburgh, PA 15236 (USA)Search for more papers by this authorThis work was performed in support of the National Energy Technology Laboratory’s ongoing research in CO2 capture under the RES contract DE-FE0004000 and has utilized neutron scattering facilities supported in part by the National Science Foundation under Agreement No. DMR-0454672. Reference in this work to any specific commercial product is to facilitate understanding and does not necessarily imply endorsement by the United States Department of Energy. Certain commercial materials and equipment are identified in this paper only to specify adequately the experimental procedure. In no case does such identification imply recommendation by NIST nor does it imply that the material or equipment identified is necessarily the best available for this purpose. The authors thank Juscelino Leao, Wendy Queen, and Craig Brown of the NIST Center for Neutron Research and Martin Green of NIST Ceramics Division for their valuable technical discussions and contributions.
Graphical Abstract
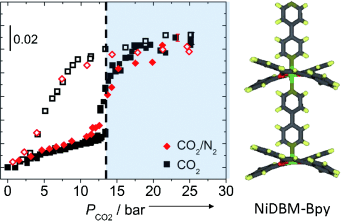
Flexibility provides selectivity: The selective adsorption of CO2 from mixtures with N2, CH4, and N2O in a dynamic porous coordination polymer (see monomer structure) was evaluated by ATR-FTIR spectroscopy, GC, and SANS. All three techniques indicate highly selective adsorption of CO2 from CO2/CH4 and CO2/N2 mixtures at 30 °C, with no selectivity observed for the CO2/N2O system.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201104130_sm_miscellaneous_information.pdf746.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ.-R. Li, R. J. Kuppler, H.-C. Zhou, Chem. Soc. Rev. 2009, 38, 1477;
- 1bD. M. D’Allesandro, B. Smit, J. R. Long, Angew. Chem. 2010, 122, 6194;
10.1002/ange.201000431 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6058.
- 2
- 2aJ.-P. Zhang, S. K. Ghoso, J.-B. Lin, S. Kitagawa, Inorg. Chem. 2009, 48, 7970;
- 2bS. Q. Ma, D. F. Sun, D. Q. Yuan, X. S. Wang, H. C. Zhou, J. Am. Chem. Soc. 2009, 131, 6445;
- 2cJ. An, R. P. Fliorella, S. J. Geib, N. L. Rosi, J. Am. Chem. Soc. 2009, 131, 8401;
- 2dB. Wang, A. P. Cote, H. Furukawa, M. O’Keeffe, O. M. Yaghi, Nature Lett. 2008, 453, 207.
- 3
- 3aE. Barea, G. Tagliabue, W. G. Wang, M. Perez-Mendoza, L. Mendez-Linan, F. J. Lopez-Garzon, S. Galli, N. Masciocchi, J. A. R. Navarro, Chem. Eur. J. 2010, 16, 931;
- 3bS. Couck, J. F. M. Denayer, G. V. Baron, T. Remy, J. Gascon, F. Kapteijn, J. Am. Chem. Soc. 2009, 131, 6326;
- 3cL. Hamon, E. Jolimaitre, G. D. Pirngruber, Ind. Eng. Chem. Res. 2010, 49, 7497;
- 3dK. Nakagawa, D. Tanaka, S. Horike, S. Shimomura, M. Higuchi, S. Kitagawa, Chem. Commun. 2010, 46, 4258;
- 3eD. Britt, H. Furukawa, B. Wang, T. G. Glover, O. M. Yaghi, Proc. Natl. Acad. Sci. USA 2009, 106, 20637;
- 3fH. Kanoh, A. Kondo, H. Noguchi, H. Kajiro, A. Tohdoh, Y. Hattori, W. C. Xu, M. Moue, T. Sugiura, K. Morita, H. Tanaka, T. Ohba, K. Kaneko, J. Colloid Interface Sci. 2009, 334, 1.
- 4
- 4aL. Hamon, P. L. Llewellyn, T. Devic, A. Ghoufi, G. Clet, V. Cuillerm, G. D. Pirngruber, G. Maurin, C. Serre, G. Dirver, W. van Beek, W. Jolimaitre, A. Vimont, M. Daturi, G. J. Ferey, J. Am. Chem. Soc. 2009, 131, 17490;
- 4bV. Finsy, L. Ma, L. Alaerts, D. E. De Vos, G. V. Baron, J. F. M. Denayer, Microporous Mesoporous Mater. 2009, 120, 221;
- 4cF.-X. Coudert, M. Jeffroy, A. H. Fuchs, A. Boutin, C. Mellot-Draznieks, J. Am. Chem. Soc. 2008, 130, 14294;
- 4dF.-X. Coudert, C. Mellot-Draznieks, A. H. Fuchs, A. Boutin, J. Am. Chem. Soc. 2009, 131, 3442;
- 4eF.-X. Coudert, C. Mellot-Draznieks, A. H. Fuchs, A. Boutin, J. Am. Chem. Soc. 2009, 131, 11329;
- 4fE. Stavitski, E. A. Pidko, S. Couck, T. Remy, E. J. M. Hensen, B. M. Weckhuysen, J. Denayer, J. Gascon, F. Kapteijn, Langmuir 2011, 27, 3970.
- 5Z. R. Herm, J. A. Swisher, B. Smit, R. Krishna, J. R. Long, J. Am. Chem. Soc. 2011, 133, 5664.
- 6Q. Y. Yang, L. Ma, C. L. Zhong, X. An, D. Liu, J. Phys. Chem. C 2011, 115, 2790.
- 7
- 7aD. V. Soldatov, J. Inclusion Phenom. Macrocyclic Chem. 2004, 48, 3;
- 7bD. V. Soldatov, G. D. Enright, C. I. Ratcliffe, A. T. Henegouwen, J. A. Ripmeester, Chem. Mater. 2001, 13, 4322;
- 7cD. V. Soldatov, G. D. Enright, J. A. Ripmeester, Chem. Mater. 2002, 14, 348;
- 7dD. V. Soldatov, G. D. Enright, J. A. Ripmeester, Cryst. Growth Des. 2004, 4, 1185;
- 7eD. V. Soldatov, I. L. Moudrakovski, C. I. Ratcliffe, R. Dutrisac, J. A. Ripmeester, Chem. Mater. 2003, 15, 4180;
- 7fD. V. Soldatov, E. A. Ukraintseva, V. A. Longvinenko, Struct. Chem. 2007, 48, 938;
- 7gD. V. Soldatov, J. A. Ripmeester, Mendeleev Commun. 2004, 3, 1.
- 8
- 8aJ. T. Culp, A. L. Goodman, D. Chirdon, S. G. Sankar, C. Matranga, J. Phys. Chem. C 2010, 114, 2184;
- 8bJ. T. Culp, M. R. Smith, E. Bittner, B. Bockrath, J. Am. Chem. Soc. 2008, 130, 12427.
- 9
- 9aP. D. C. Dietzel, R. E. Johnsen, H. Fjellvag, S. Bordiga, E. Groppo, S. Chavan, R. Blom, Chem. Commun. 2008, 5125;
- 9bS. Natesakhawat, J. T. Culp, C. Matranga, B. Bockrath, J. Phys. Chem. C 2007, 111, 1055;
- 9cA. L. Goodman, L. A. Campus, K. T. Schroeder, Energy Fuels 2005, 19, 471;
- 9dS. G. Kazarian, M. F. Vincent, F. V. Bright, C. L. Liotta, C. A. Eckert, J. Am. Chem. Soc. 1996, 118, 1729;
- 9eM. R. Nelson, R. F. Borkman, J. Phys. Chem. A 1998, 102, 7860;
- 9fS. P. Nalawade, F. Picchioni, L. Janssen, D. W. Grijpma, J. Feijen, J. Appl. Polym. Sci. 2008, 109, 3376;
- 9gC. Serre, S. Bourrelly, A. Vimont, N. A. Ramsahye, G. Maurin, P. L. Llewellyn, M. Daturi, Y. Filinchuk, O. Leynaud, P. Barnes, G. Férey, Adv. Mater. 2007, 19, 2246;
- 9hA. Vimont, A. Travert, P. Bazin, J. C. Lavalley, M. Daturi, C. Serre, G. Ferey, S. Bourrelly, P. L. Llewellyn, Chem. Commun. 2007, 3291;
- 9iL. Heinke, D. Tzoulaki, C. Chmelik, F. Hibbe, B. J. M. van, H. Lim, J. Li, R. Krishna, J. Karger, Phys. Rev. Lett. 2009, 102, 065901.
- 10D. R. Lide, Handbook of Chemistry and Physics, 74 ed., CRC, Ann Arbor, 1994.
- 11C. J. Glinka, J. G. Barker, B. Hammouda, S. Kreuger, J. J. Moyer, W. J. Orts, J. Appl. Crystallogr. 1998, 31, 430.
- 12
- 12aR. M. Barrer, J. Phys. Chem. Solids 1960, 16, 84;
- 12bR. M. Barrer, Philos. Trans. R. Soc. London Ser. A 1984, 311, 333;
- 12cR. M. Barrer, Pure Appl. Chem. 1989, 61, 1903.
- 13
- 13aF. Salles, G. Maurin, C. Serre, P. L. Llewellyn, C. Knöfel, H. J. Choi, Y. Filinchuk, L. Oliviero, A. Vimont, J. R. Long, G. Férey, J. Am. Chem. Soc. 2010, 132, 13782;
- 13bD. Fairen-Jimenez, N. A. Seaton, T. Düren, Langmuir 2010, 26, 14694;
- 13cA. V. Neimark, F.-X. Coudert, A. Boutin, A. H. Fuchs, J. Phys. Chem. Lett. 2010, 1, 445;
- 13dA. Ghoufi, G. Maurin, J. Phys. Chem. C 2010, 114, 6496;
- 13eA. Ghoufi, G. Maurin, G. Ferey, J. Phys. Chem. Lett. 2010, 1, 2810.