syn-Selective Rhodium(I)-Catalyzed Allylations of Ketimines Proceeding through a Directed CH Activation/Allene Addition Sequence†
Duc N. Tran
Laboratory of Organic Chemistry, ETH Zurich, Wolfgang-Pauli-Strasse 10, HCI H 304, 8093 Zurich (Switzerland), Fax: (+41) 44-632-1328 http://www.cramer.ethz.ch
Search for more papers by this authorDr. Nicolai Cramer
Laboratory of Organic Chemistry, ETH Zurich, Wolfgang-Pauli-Strasse 10, HCI H 304, 8093 Zurich (Switzerland), Fax: (+41) 44-632-1328 http://www.cramer.ethz.ch
Search for more papers by this authorDuc N. Tran
Laboratory of Organic Chemistry, ETH Zurich, Wolfgang-Pauli-Strasse 10, HCI H 304, 8093 Zurich (Switzerland), Fax: (+41) 44-632-1328 http://www.cramer.ethz.ch
Search for more papers by this authorDr. Nicolai Cramer
Laboratory of Organic Chemistry, ETH Zurich, Wolfgang-Pauli-Strasse 10, HCI H 304, 8093 Zurich (Switzerland), Fax: (+41) 44-632-1328 http://www.cramer.ethz.ch
Search for more papers by this authorWe thank Dr. B. Schweizer for X-ray crystallographic analysis of compound 6 ce and the ETH Zurich (ETH-16 08-3) for funding. We are grateful to Solvias AG for MeOBiphep ligands, Takasago International Corporation for Segphos, and Umicore AG & Co. KG for rhodium salts. N.C. thanks the Fonds der Chemischen Industrie for a Liebig Fellowship.
Graphical Abstract
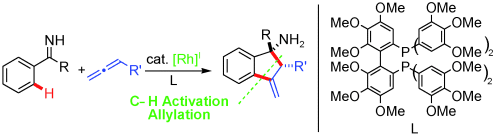
On a Rh-oll: Rhodium(I)-catalyzed CH activations of ketimines and subsequent carborhodation of an allene led to an allyl metal species, which then allylated the imine directing group to give highly functionalized methylene dihydroindenyl amines (see scheme) with excellent regio- and diastereoselectivity.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201004179_sm_miscellaneous_information.pdf3.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For representative reviews on CH bond functionalizations, see:
- 1aG. Dyker, Angew. Chem. 1999, 111, 1808–1822;
10.1002/(SICI)1521-3757(19990614)111:12<1808::AID-ANGE1808>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1698–1712;10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 1bC. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 2001, 34, 633–639;
- 1cV. Ritleng, C. Sirlin, M. Pfeffer, Chem. Rev. 2002, 102, 1731–1769;
- 1dF. Kakiuchi, N. Chatani, Adv. Synth. Catal. 2003, 345, 1077–1101;
- 1eK. Godula, D. Sames, Science 2006, 312, 67–72;
- 1fF. Kakiuchi, Top. Organomet. Chem. 2007, 24, 1–33;
- 1gT. Satoh, M. Miura, Top. Organomet. Chem. 2007, 24, 61–84;
- 1hI. V. Seregin, V. Gevorgyan, Chem. Soc. Rev. 2007, 36, 1173–1193;
- 1iD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174–238;
- 1jF. Kakiuchi, T. Kochi, Synthesis 2008, 3013–3039;
- 1kJ. C. Lewis, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2008, 41, 1013–1025;
- 1lX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196–5217; Angew. Chem. Int. Ed. 2009, 48, 5094–5115.
- 2For reviews on directed CH bond functionalizations, see:
- 2aF. Kakiuchi, S. Murai, Acc. Chem. Res. 2002, 35, 826–834;
- 2bL. Ackermann, Top. Organomet. Chem. 2007, 24, 35–60;
- 2cD. Kalyani, M. S. Sanford, Top. Organomet. Chem. 2007, 24, 85–116;
- 2dC.-H. Jun, E.-A. Jo, J.-W. Park, Eur. J. Org. Chem. 2007, 1869–1881;
- 2eY. J. Park, J.-W. Park, C.-H. Jun, Acc. Chem. Res. 2008, 41, 222–234;
- 2fD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624–655.
- 3
- 3aK. Ueura, T. Satoh, M. Miura, J. Org. Chem. 2007, 72, 5362–5367;
- 3bK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407–1409.
- 4
- 4aS.-G. Lim, J. Hee Lee, C. W. Moon, J.-B. Hong, C.-H. Jun, Org. Lett. 2003, 5, 2759–2761;
- 4bN. Guimond, K. Fagnou, J. Am. Chem. Soc. 2009, 131, 12050–12051;
- 4cT. Fukutani, N. Umeda, K Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141–5143.
- 5
- 5aY. Kuninobu, A. Kawata, K. Takai, J. Am. Chem. Soc. 2005, 127, 13498–13499;
- 5bY. Kuninobu, Y. Tokunaga, A. Kawata, K. Takai, J. Am. Chem. Soc. 2006, 128, 202–209;
- 5cY. Kuninobu, Y. Nishina, M. Shouho, K. Takai, Angew. Chem. 2006, 118, 2832–2834;
10.1002/ange.200503627 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2766–2768.
- 6Z.-M. Sun, S.-P. Chen, P. Zhao, Chem. Eur. J. 2010, 16, 2619–2627.
- 7
- 7aE. Skucas, J. F. Bower, M. J. Krische, J. Am. Chem. Soc. 2007, 129, 12678–12679;
- 7bM.-Y. Ngai, E. Skucas, M. J. Krische, Org. Lett. 2008, 10, 2705–2708;
- 7cS. B. Han, I. S. Kim, H. Han, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 6916–6917;
- 7dE. Skucas, J. R. Zbieg, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 5054–5055.
- 8For a review, see:
- 8aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242–3272; for contributions from our own laboratory, see:
- 8bT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. 2009, 121, 6438–6441;
10.1002/ange.200903189 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6320–6323;
- 8cM. Albicker, N. Cramer, Angew. Chem. 2009, 121, 9303–9306;
10.1002/ange.200905060 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9139–9142.
- 9For some mechanistic and theoretical studies, see:
- 9aY. Boutadla, D. L. Davies, S. A. Macgregor, A. I. Poblador-Bahamonde, Dalton Trans. 2009, 5820–5831;
- 9bL. Li, W. W. Brennessel, W. D. Jones, Organometallics 2009, 28, 3492–3500;
- 9cD. L. Strout, S. Zaric, S. Q. Niu and M. B. Hall, J. Am. Chem. Soc. 1996, 118, 6068–6069;
- 9dM. D. Su, S. Y. Chu, J. Am. Chem. Soc. 1997, 119, 5373–5383;
- 9eS. Q. Niu, M. B. Hall, J. Am. Chem. Soc. 1998, 120, 6169–6170;
- 9fA. J. Toner, S. Grundemann, E. Clot, H. H. Limbach, B. Donnadieu, S. Sabo-Etienne, B. Chaudret, J. Am. Chem. Soc. 2000, 122, 6777–6778.
- 10
- 10aR. Zimmer, C. U. Dinesh, E. Nandanan, F. A. Khan, Chem. Rev. 2000, 100, 3067–3126;
- 10bS. Ma, Chem. Rev. 2005, 105, 2829–2872.
- 11
- 11aP. A. Evans, J. D. Nelson, J. Am. Chem. Soc. 1998, 120, 5581–5582;
- 11bP. A. Evans, J. D. Nelson, Tetrahedron Lett. 1998, 39, 1725–1728.
- 12Unsubstituted ketimines are readily available by the addition of lithium or Grignard reagents to nitriles or from the ketones by a treatment with ammonia and titanium tetrachloride. The purity of the imines 1 is of great importance for the conversion of the reaction. Substituted diaryl ketimines (NBn, NTs, NAc, NCO2Me) give the corresponding product 6 only very low yields.
- 13The removal of the aromatic hydrogen atom from the rhodium complex as water, or acetic acid seems to play an important role in the generation of a reactive cyclometalated and/or allylic species.
- 14S. Jeulin, S. Duprat de Paule, V. Ratovelomanana-Vidal, J.-P. Genet, N. Champion, P. Dellis, Proc. Natl. Acad. Sci. USA 2004, 101, 5799–5804.
- 15The carbonyl stretching frequency of the corresponding [LRh(Cl)CO] complex is a convenient indicator for the relative π acidity of the phosphines ligands: L1: ν=2004 cm−1, L2: ν=2017 cm−1, L4: ν=1984 cm−1, L5: ν=2000 cm−1, binap: ν=2006 cm−1 (data obtained on a Varian 640-IR FT-IR spectrometer).
- 16The allMeOBiphep ligand (L5) was developed at F. Hoffmann-La Roche AG: EP0926151A1. However, its performance in catalytic reactions has never been disclosed. rac-L5 is conveniently prepared in two steps from tris-3,4,5-trimethoxyphosphineoxide in 59 % yield.
- 17The obtained e.r. values for 6 aa were: 57:43 ((R)-binap), 60:40 (L1), 71:29 (L3).
- 18E. Clot, M. Besora, F. Maseras, C. Mégret, O. Eisenstein, B. Oelckers, R. N. Perutz, Chem. Commun. 2003, 490–491.
- 19Crystallographic data for 6 ce: C18H13F2NO, M=297.30, monoclinic, space group C2/c, a=25.9672(6) Å, b=7.3668(2) Å, c=17.6121(5) Å, β=124.3779(13)°, V=2780.63(13) Å3, Z=8, Dcalc=1.420 Mg m−3, T=123 K, reflections collected: 5596, independent reflections: 3183 (Rint=0.046), R(all)=0.0660, wR(gt)=0.1334. CCDC 779690 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20The absolute configuration of (−)-6 ge has not yet been determined.