Tandem Catalytic Asymmetric Friedel–Crafts/Henry Reaction: Control of Three Contiguous Acyclic Stereocenters†
Correction(s) for this article
-
Tandem Catalytic Asymmetric Friedel–Crafts/Henry Reaction: Control of Three Contiguous Acyclic Stereocenters
- Volume 49Issue 50Angewandte Chemie International Edition
- pages: 9555-9555
- First Published online: December 7, 2010
Takayoshi Arai Prof. Dr.
Department of Chemistry, Graduate School of Science, Chiba University, Inage, Chiba 263-8522 (Japan), Fax: (+81) 43-290-2889 http://synthesis.chem.chiba-u.jp/
Search for more papers by this authorNaota Yokoyama
Department of Chemistry, Graduate School of Science, Chiba University, Inage, Chiba 263-8522 (Japan), Fax: (+81) 43-290-2889 http://synthesis.chem.chiba-u.jp/
Search for more papers by this authorTakayoshi Arai Prof. Dr.
Department of Chemistry, Graduate School of Science, Chiba University, Inage, Chiba 263-8522 (Japan), Fax: (+81) 43-290-2889 http://synthesis.chem.chiba-u.jp/
Search for more papers by this authorNaota Yokoyama
Department of Chemistry, Graduate School of Science, Chiba University, Inage, Chiba 263-8522 (Japan), Fax: (+81) 43-290-2889 http://synthesis.chem.chiba-u.jp/
Search for more papers by this authorThis research was supported by the Industrial Technology Research Grant Program in 2006 from the New Energy and Industrial Technology Development Organization (NEDO) of Japan and by a Grant-in Aid for Scientific Research on Priority Areas (No. 19028007, “Chemistry of Concerto Catalysis”) from the Ministry of Education, Culture, Sports, Science and Technology (Japan). We thank Prof. Akira Yanagisawa at Chiba University for helpful discussions.
Graphical Abstract
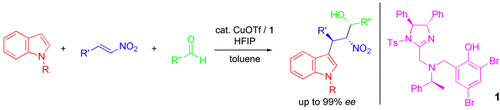
Good things come in threes: Highly functionalized indole derivatives with three contiguous stereocenters were formed in the title reaction of an indole, a nitroalkene, and an aldehyde with the imidazoline–aminophenol catalyst 1–CuOTf in the presence of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). The major isomers of the adducts were obtained with up to 99 % ee. Tf=trifluoromethanesulfonyl, Ts=p-toluenesulfonyl; R=H, Me; R′,R′′=alkyl, aryl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200801373_sm_miscellaneous_information.pdf5.9 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. C. Nicolaou, T. Montagnon, S. A. Snyder, Chem. Commun. 2003, 551–564;
- 1bJ.-C. Wasilke, S. J. Obrey, R. T. Baker, G. C. Bazan, Chem. Rev. 2005, 105, 1001–1020;
- 1cD. J. Ramón, M. Yus, Angew. Chem. 2005, 117, 1628–1661;
10.1002/ange.200460548 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1602–1634;
- 1dD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1590–1601;
10.1002/ange.200603129 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1570–1581.
- 2S. L. Schreiber, Science 2000, 287, 1964–1969.
- 3For controlling of over three stereocenters in cascade asymmetric catalysis, see:
- 3aD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861–863;
- 3bD. Enders, M. R. M. Hüttl, J. Runsink, G. Raabe, B. Wendt, Angew. Chem. 2007, 119, 471–473;
10.1002/ange.200603434 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 467–469;
- 3cY. Hayashi, T. Okano, S. Aratake, D. Hazelard, Angew. Chem. 2007, 119, 5010–5013; Angew. Chem. Int. Ed. 2007, 46, 4922–4925;
- 3dE. Reyes, H. Jiang, A. Milelli, P. Elsner, R. G. Hazell, K. A. Jørgensen, Angew. Chem. 2007, 119, 9362–9365;
10.1002/ange.200704454 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 9202–9205;
- 3eL. Zu, J. Wang, H. Li, H. Xie, W. Jiang, W. Wang, J. Am. Chem. Soc. 2007, 129, 1036–1037;
- 3fL. Zu, H. Li, H. Xie, J. Wang, W. Jiang, Y. Tang, W. Wang, Angew. Chem. 2007, 119, 3806–3808;
10.1002/ange.200700485 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3732–3734;
- 3gJ. Wang, H. Xie, H. Li, L. Zu, W. Wang, Angew. Chem. 2008, 120, 4245–4247; Angew. Chem. Int. Ed. 2008, 47, 4177–4179.
- 4T. Arai, N. Yokoyama, A. Yanagisawa, Chem. Eur. J. 2008, 14, 2052–2059.
- 5For selected examples of pioneering studies on the catalytic asymmetric Henry reaction, see:
- 5aH. Sasai, T. Suzuki, S. Arai, T. Arai, M. Shibasaki, J. Am. Chem. Soc. 1992, 114, 4418–4420;
- 5bB. M. Trost, V. S. C. Yeh, Angew. Chem. 2002, 114, 889–891;
10.1002/1521-3757(20020301)114:5<889::AID-ANGE889>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 861–863;10.1002/1521-3773(20020301)41:5<861::AID-ANIE861>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 5cC. Palomo, M. Oiarbide, A. Laso, Angew. Chem. 2005, 117, 3949–3952;
10.1002/ange.200463075 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3881–3884;
- 5dY. Kogami, T. Nakajima, T. Ashizawa, S. Kezuka, T. Ikeno, T. Yamada, Chem. Lett. 2004, 33, 614–615;
- 5eB. M. Choudary, K. V. S. Ranganath, U. Pal, M. L. Kantam, B. Sreedhar, J. Am. Chem. Soc. 2005, 127, 13167–13171;
- 5fT. Ooi, K. Doda, K. Maruoka, J. Am. Chem. Soc. 2003, 125, 2054–2055;
- 5gT. Marcelli, R. N. S. van der Haas, J. H. van Maarseveen, H. Hiemstra, Angew. Chem. 2006, 118, 943–945;
10.1002/ange.200503724 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 929–931;
- 5hY. Sohtome, Y. Hashimoto, K. Nagasawa, Eur. J. Org. Chem. 2006, 2894–2897.
- 6For Cu-catalyzed enantioselective Henry reactions, see:
- 6aC. Christensen, K. Juhl, K. A. Jørgensen, Chem. Commun. 2001, 2222–2223;
- 6bD. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw, C. W. Downey, J. Am. Chem. Soc. 2003, 125, 12692–12693;
- 6cT. Arai, M. Watanabe, A. Fujiwara, N. Yokoyama, A. Yanagisawa, Angew. Chem. 2006, 118, 6124–6127;
10.1002/ange.200602255 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5978–5981.
- 7For enantioselective Friedel–Crafts reactions with indole, see:
- 7aR. P. Herrera, V. Sgarzani, L. Bernardi, A. Ricci, Angew. Chem. 2005, 117, 6734–6737;
10.1002/ange.200500227 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6576–6579;
- 7bS.-F. Lu, D.-M. Du, J. Xu, Org. Lett. 2006, 8, 2115–2118;
- 7cY.-X. Jia, S.-F. Zhu, Y. Yang, Q.-L. Zhou, J. Org. Chem. 2006, 71, 75–80.
- 8For examples of catalytic asymmetric Michael–aldol reactions, see:
- 8aT. Arai, H. Sasai, K. Aoe, K. Okamura, T. Date, M. Shibasaki, Angew. Chem. 1996, 108, 103–105;
10.1002/ange.19961080123 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 104–106;
- 8bB. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos, A. H. M. de Vries, Angew. Chem. 1997, 109, 2733–2736; Angew. Chem. Int. Ed. Engl. 1997, 36, 2620–2623;
- 8cS. J. Taylor, M. O. Duffey, J. P. Morken, J. Am. Chem. Soc. 2000, 122, 4528–4529;
- 8dK. Yoshida, M. Ogasawara, T. Hayashi, J. Am. Chem. Soc. 2002, 124, 10984–10985;
- 8eD. F. Cauble, J. D. Gipson, M. J. Krische, J. Am. Chem. Soc. 2003, 125, 1110–1111;
- 8fO. Chuzel, J. Deschamp, C. Chausteur, O. Riant, Org. Lett. 2006, 8, 5943–5946;
- 8gH. Sundén, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Chem. Eur. J. 2007, 13, 574–581;
- 8hA. Carlone, S. Cabrera, M. Marigo, K. A. Jørgensen, Angew. Chem. 2007, 119, 1119–1122; Angew. Chem. Int. Ed. 2007, 46, 1101–1104;
- 8iJ. Wang, H. Li, H. Xie, L. Zu, X. Shen, W. Wang, Angew. Chem. 2007, 119, 9208–9211; Angew. Chem. Int. Ed. 2007, 46, 9050–9053; see also:
- 8jD. B. Ramachary, N. S. Chowdari, C. F. Barbas III, Angew. Chem. 2003, 115, 4365–4369;
10.1002/ange.200351916 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4233–4237;
- 8kJ. W. Yang, M. T. H. Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036–15037;
- 8lY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051–15053.