Enhanced π Conjugation around a Porphyrin[6] Nanoring†
Markus Hoffmann
Department of Chemistry, Oxford University, Chemistry Research Laboratory, 12 Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-28-5002 http://users.ox.ac.uk/∼hlagroup
Search for more papers by this authorJoakim Kärnbratt
Department of Chemical and Biological Engineering, Physical and Organic Chemistry, Chalmers University of Technology, Kemivägen 10, 412 96 Göteborg (Sweden)
Search for more papers by this authorBo Albinsson Prof.
Department of Chemical and Biological Engineering, Physical and Organic Chemistry, Chalmers University of Technology, Kemivägen 10, 412 96 Göteborg (Sweden)
Search for more papers by this authorHarry L. Anderson Prof.
Department of Chemistry, Oxford University, Chemistry Research Laboratory, 12 Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-28-5002 http://users.ox.ac.uk/∼hlagroup
Search for more papers by this authorMarkus Hoffmann
Department of Chemistry, Oxford University, Chemistry Research Laboratory, 12 Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-28-5002 http://users.ox.ac.uk/∼hlagroup
Search for more papers by this authorJoakim Kärnbratt
Department of Chemical and Biological Engineering, Physical and Organic Chemistry, Chalmers University of Technology, Kemivägen 10, 412 96 Göteborg (Sweden)
Search for more papers by this authorBo Albinsson Prof.
Department of Chemical and Biological Engineering, Physical and Organic Chemistry, Chalmers University of Technology, Kemivägen 10, 412 96 Göteborg (Sweden)
Search for more papers by this authorHarry L. Anderson Prof.
Department of Chemistry, Oxford University, Chemistry Research Laboratory, 12 Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-28-5002 http://users.ox.ac.uk/∼hlagroup
Search for more papers by this authorThis work was supported by EPSRC. We thank Johannes K. Sprafke for preliminary experiments on the synthesis of the template, and the EPSRC Mass Spectrometry Service (Swansea) for mass spectra.
Graphical Abstract
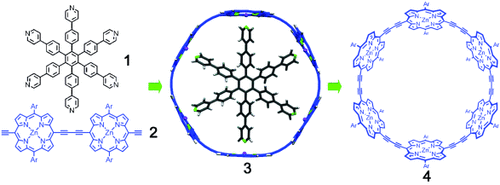
Strong cycle: The cyclic hexamer–template complex 3 obtained through template-directed trimerization of a porphyrin dimer 2, using a hexapyridyl template 1, is extremely stable (Kf=7×1038 M−1), but the free macrocycle 4 can be liberated using amine ligands. Spectroscopic data and DFT calculations show that the cyclic hexamer is more conjugated than its linear analogue.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200801188_sm_miscellaneous_information.pdf1.7 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews see:
- 1aT. Kawase, H. Kurata, Chem. Rev. 2006, 106, 5250–5273;
- 1bK. Tahara, Y. Tobe, Chem. Rev. 2006, 106, 5274–5290;
- 1cL. T. Scott, Angew. Chem. 2003, 115, 4265–4267; Angew. Chem. Int. Ed. 2003, 42, 4133–4135.
- 2T. Kawase, H. R. Darabi, M. Oda, Angew. Chem. 1996, 108, 2803–2805;
10.1002/ange.19961082214 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2664–2666.
- 3M. Ohkita, K. R. Ando, T. Tsuji, Chem. Commun. 2001, 2570–2571.
- 4
- 4aS. Anderson, H. L. Anderson, J. K. M. Sanders, Acc. Chem. Res. 1993, 26, 469–475;
- 4bS. Rucareanu, A. Schuwey, A. Gossauer, J. Am. Chem. Soc. 2006, 128, 3396–3413;
- 4cJ. Li, A. Ambroise, S. I. Yang, J. R. Diers, J. Seth, C. R. Wack, D. F. Bocian, D. Holten, J. S. Lindsey, J. Am. Chem. Soc. 1999, 121, 8927–8940;
- 4dY. Kuramochi, A. Satake, Y. Kobuke, J. Am. Chem. Soc. 2004, 126, 8668–8669;
- 4eT. Hori, N. Aratani, A. Takagi, T. Matsumoto, T. Kawai, M.-C. Yoon, Z. S. Yoon, S. Cho, D. Kim, A. Osuka, Chem. Eur. J. 2006, 12, 1319–1327.
- 5M. Hoffmann, C. J. Wilson, B. Odell, H. L. Anderson, Angew. Chem. 2007, 119, 3183–3186;
10.1002/ange.200604601 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3122–3125.
- 6R. Rathore, C. L. Burns, M. I. Deselnicu, Org. Lett. 2001, 3, 2887–2890.
- 7
- 7aJ. A. Marsden, J. J. Miller, M. M. Haley, Angew. Chem. 2004, 116, 1726–1729;
10.1002/ange.200353043 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1694–1697;
- 7bA. S. Batsanov, J. C. Collings, I. J. S. Fairlamb, J. P. Holland, J. A. K. Howard, Z. Lin, T. B. Marder, A. C. Parsons, R. M. Ward, J. Zhu, J. Org. Chem. 2005, 70, 703–706.
- 8
- 8aP. Ballester, A. I. Oliva, A. Costa, P. M. Deya, A. Frontera, R. M. Gomila, C. A. Hunter, J. Am. Chem. Soc. 2006, 128, 5560–5569;
- 8bV. M. Krishnamurthy, V. Semetey, P. J. Bracher, N. Shen, G. M. Whitesides, J. Am. Chem. Soc. 2007, 129, 1312–1320;
- 8cG. Ercolani, Struct. Bonding (Berlin) 2006, 121, 167–215.
- 9S. J. Strickler, R. A. Berg, J. Chem. Phys. 1962, 37, 814–822.
- 10The integrated absorptivity of the most red-shifted component of the Q-band was calculated from an estimate of the width of the band. Since this transition overlaps heavily with the rest of the Q-band, this estimate may be inaccurate but it is good enough for the qualitative analysis presented here.
- 11
- 11aG. McDermott, S. M. Prince, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Papiz, R. J. Cogdell, N. W. Isaacs, Nature 1995, 374, 517–521;
- 11bY. Nakamura, N. Aratani, A. Osuka, Chem. Soc. Rev. 2007, 36, 831–845.