Modulating the Lewis Acidity of Boron Using a Photoswitch†
Vincent Lemieux
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorM. Daniel Spantulescu
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorKim K. Baldridge Prof.
Organic Chemistry Institute, University of Zürich, Winterthurerstrasse 190, 8050 Zürich (Switzerland)
Search for more papers by this authorNeil R. Branda Prof.
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorVincent Lemieux
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorM. Daniel Spantulescu
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorKim K. Baldridge Prof.
Organic Chemistry Institute, University of Zürich, Winterthurerstrasse 190, 8050 Zürich (Switzerland)
Search for more papers by this authorNeil R. Branda Prof.
4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6 (Canada), Fax: (+1) 778-782-8061
Search for more papers by this authorThis work was supported by the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chair Program, Simon Fraser University and the University of Zürich. K.K.B. would like to acknowledge the Swiss National Science Foundation for support of this work, and Donald Truhlar for enabling the use of the M06-2X functional recently developed but not yet in the public domain.
Graphical Abstract
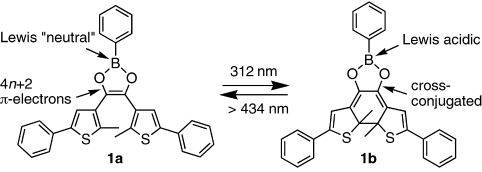
Light turns the Lewis acid on: The Lewis acidity of a boron atom integrated into a cyclic dithienylethene photoswitch is modulated by light: 1 a has low Lewis acidity since the p orbital of the boron center is partially occupied by delocalized π electrons, whereas the rearrangement of the π electrons in 1 b reduces the electron density at the boron center and turns the Lewis acid “on”.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200800869_sm_miscellaneous_information.pdf1.7 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Ishihara, H. Yamamoto, Eur. J. Org. Chem. 1999, 527–538.
10.1002/(SICI)1099-0690(199903)1999:3<527::AID-EJOC527>3.0.CO;2-R CAS Web of Science® Google Scholar
- 2E. Y.-X. Chen, T. J. Marks, Chem. Rev. 2000, 100, 1391–1434.
- 3G. L. Jialanella, T. Ristoski, US Patent 7,247,596, July 24, 2007, and references therein.
- 4S. Yamaguchi, A. Wakamiya, Pure Appl. Chem. 2006, 78, 1413–1424.
- 5
- 5a Molecular Switches (Ed.: ), Wiley-VCH, Weinheim, 2001;
- 5b Photochromism Molecules and Systems (Eds.: ), Elsevier, Amsterdam, 2003;
- 5c Organic Photochromic and Thermochromic Compounds (Eds.: ), Plenum, New York, 1999.
- 6
- 6aSpecial issue on photochromism: M. Irie, Chem. Rev. 2000, 100, 1685–1716;
- 6bM. Irie in Molecular Switches (Ed.: ), Wiley-VCH, Weinheim, 2001, pp. 37–62;
- 6cM. Irie in Photochromic and Thermochromic Compounds, Vol. 1 (Eds.: ), Plenum, New York, 1999, pp. 207–222;
- 6dH. Tian, S. Yang, Chem. Soc. Rev. 2004, 33, 85–97;
- 6eH. Tian, S. Wang, Chem. Commun. 2007, 781–792.
- 7
- 7aS. H. Kawai, S. L. Gilat, J.-M. Lehn, Eur. J. Org. Chem. 1999, 2359–2366;
10.1002/(SICI)1099-0690(199909)1999:9<2359::AID-EJOC2359>3.0.CO;2-# CAS Web of Science® Google Scholar
- 7bY. Odo, K. Matsuda, M. Irie, Chem. Eur. J. 2006, 12, 4283–4288;
- 7cD. Sud, T. B. Norsten, N. R. Branda, Angew. Chem. 2005, 117, 2055–2057;
10.1002/ange.200462538 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2019–2021;
- 7dH. D. Samachetty, N. R. Branda, Chem. Commun. 2005, 2840–2842;
- 7eH. D. Samachetty, N. R. Branda, Pure Appl. Chem. 2006, 78, 2351–2359.
- 8
- 8aV. Lemieux, N. R. Branda, Org. Lett. 2005, 7, 2969–2972;
- 8bV. Lemieux, S. Gauthier, N. R. Branda, Angew. Chem. 2006, 118, 6974–6978;
10.1002/ange.200601584 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6820–6824;
- 8cD. Sud, T. J. Wigglesworth, N. R. Branda, Angew. Chem. 2007, 119, 8163–8165;
10.1002/ange.200703034 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8017–8019.
- 9Although the photoswitching of the Lewis acidity of a catecholborane by an azobenzene that reversibly varied the number of coordinating ligands was recently demonstrated (N. Kano, J. Yoshino, T. Kawashima, Org. Lett. 2005, 7, 3909–3911), it is plagued by severe limitations such as slow photoisomerization (2 h to reach the photostationary state) and low conversion (only 51 % of the photoisomeric product is formed). These limitations, as well as the thermal instability often observed for azobenzene derivatives, are not usually suffered by dithienylethene derivatives. Another limitation is based on the fact that the operation of the azobenzene system relies on changing the coordination number at the boron center using a photoresponsive ligand. This implies that if a Lewis base stronger than the azobenzene is present, it will displace the photoswitch.
- 10
- 10aR. L. Letsinger, S. B. Hamilton, J. Org. Chem. 1960, 25, 592–595;
- 10bG. Smolinsky, J. Org. Chem. 1961, 26, 4915–4917.
- 11See Supporting Information for details.
- 12S. N. Ivanov, B. V. Lichitskii, A. A. Dudinov, A. Y. Martynkin, M. M. Krayushkin, Chem. Heterocycl. Compd. 2001, 37, 85–90.
- 13J. P. Girault, P. Scribe, G. Dana, Tetrahedron 1973, 29, 413–418.
- 14R. G. Kidd in NMR of Newly Accessible Nuclei, Vol. 2 (Ed.: ), Academic Press, New York, 1983.
- 15All ring-closing reactions were carried out using the light source from a lamp used for visualizing TLC plates at 312 nm (Spectroline E series, 470 W cm−2). The ring-opening reactions were carried out using the light of a 300 W halogen photo-optic source passed through a 434 nm cutoff filter to eliminate higher energy light.
- 16It was immediately observed that the ring-closed photoisomer 1 b is significantly more sensitive to oxidation than its ring-open counterpart. All irradiation studies were performed in anhydrous and oxygen-free atmosphere.
- 17A 1:1 mixture of 1 a and 1 b was used to monitor the changes in chemical shift for the ring-closed isomer. This mixture was obtained by irradiating a solution of 1 a with 312 nm light for 10 min.