DNA-Based Catalytic Enantioselective Michael Reactions in Water†
David Coquière Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorBen L. Feringa Prof. Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorGerard Roelfes Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorDavid Coquière Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorBen L. Feringa Prof. Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorGerard Roelfes Dr.
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands, Fax: (+31) 50-363-4296
Search for more papers by this authorWe thank A. J. Boersma for useful discussions and for providing the substrates, and the NRSC-Catalysis for financial support.
Graphical Abstract
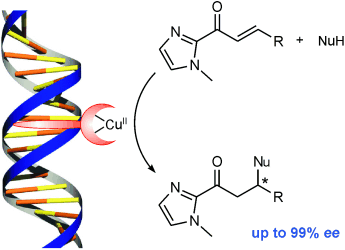
High, but not dry: A highly enantioselective Michael reaction in water has been developed by using a simple DNA-based catalyst. Enantioselectivities of up to 99 % ee could be obtained by using nitromethane and dimethyl malonate as the nucleophiles and α,β-unsaturated 2-acylimidazoles as the Michael acceptors. The reactions can be performed on a preparative scale and the catalyst can be recycled.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z703459_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC.-J. Li, T.-H. Chan, Organic Reactions in Aqueous Media, Wiley-VCH, New York, 1997;
- 1bB. Cornils, W. A. Herrmann, Aqueous-Phase Organometallic Catalysis, Wiley-VCH, Weinheim, 1998;
- 1cC.-J. Li, T.-H. Chan, Organic Synthesis in Water (Ed.: ), Blackie Academic and Professional, London, 1998;
- 1dN. Krause, A. Hoffman-Röder, Synthesis 2001, 56, 8033–8061;
- 1eC.-J. Li, Chem. Rev. 2005, 105, 3095–3166;
- 1fS. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb, K. B. Sharpless, Angew. Chem. 2005, 117, 3339–3343; Angew. Chem. Int. Ed. 2005, 44, 3275–3279;
- 1gJ. B. F. N. Engberts in Organic Reactions in Water; Principles, Strategies and Applications (Ed.: ), Blackwell, Oxford, 2007, pp. 29–91.
10.1002/9780470988817.ch2 Google Scholar
- 2For reviews on stereoselective organic reactions in aqueous media, see
- 2aD. Sinou, Adv. Synth. Catal. 2002, 344, 221–237;
- 2bU. M. Lindström, Chem. Rev. 2002, 102, 2751–2772;
- 2cK. Manabe, S. Kobayashi, Chem. Eur. J. 2002, 8, 4094–4101.
10.1002/1521-3765(20020916)8:18<4094::AID-CHEM4094>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 3
- 3aE. Keller, B. L. Feringa, Tetrahedron Lett. 1996, 37, 1879–1882;
- 3bE. Keller, B. L. Feringa, Synlett 1997, 842–844;
- 3cR. Ding, K. Katebzadeh, L. Roman, K.-E. Bergquist, U. M. Lindström, J. Org. Chem. 2006, 71, 352–355;
- 3dK. Aplander, R. Ding, U. M. Lindström, J. Wennerberg, S. Schultz, Angew. Chem. 2007, 119, 4627–4630;
10.1002/ange.200700560 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4543–4546.
- 4S. Kobayashi, K. Kakumoto, Y. Mori, K. Manabe, Isr. J. Chem. 2001, 41, 247–249.
- 5Y. Hamashima, D. Hotta, N. Umebayashi, Y. Tsuchiya, T. Suzuki, M. Sodeoka, Adv. Synth. Catal. 2005, 347, 1576–1586.
- 6Examples of enantioselective aryl or vinyl organometallic conjugate additions catalyzed by rhodium complexes in aqueous media have been described:
- 6aT. S. Huang, C. J. Li, Org. Lett. 2001, 3, 2037–2039;
- 6bT. S. Huang, Y. Meng, S. Venkatraman, D. Wang, C. J. Li, J. Am. Chem. Soc. 2001, 123, 7451–7452;
- 6cM. Lautens, J. Mancuso, Org. Lett. 2002, 4, 2105–2108;
- 6dQ. Shi, L. Xu, X. Li, X. Jia, R. Wang, T. T.-L. Au-Yeung, A. S. C. Chan, T. Hayashi, R. Cao and M. Hong, Tetrahedron Lett. 2003, 44, 6505–6508;
- 6eR. B. C. Jagt, J. G. De Vries, B. L. Feringa, A. J. Minnaard, Org. Lett. 2005, 7, 2433–2435;
- 6fS. Källström, R. B. C. Jagt, R. Sillanpää, B. L. Feringa, A. J. Minnaard, R. Leino, Eur. J. Org. Chem. 2006, 3826–3833.
- 7Several examples of enantioselective organocatalytic Michael additions to nitroalkenes in water have been reported:
- 7aN. Mase, K. Watanabe, H. Yoda, K. Takabe, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 4966–4967;
- 7bS. Luo, X. Mi, S. Liu, H. Xu, J. P. Cheng, Chem. Commun. 2006, 3687–3689;
- 7cZ. Y. Yan, Y. N. Niu, H. L. Wei, L. Y. Wu, Y. B. Zhao, Y. M. Liang, Tetrahedron: Asymmetry 2006, 17, 3288–3293;
- 7dE. Alza, X. C. Cambeiro, C. Jimeno, M. A. Pericàs, Org. Lett. 2007, 9, 1943–1946;
- 7eV. Singh, V. K. Singh, Org. Lett. 2007, 9, 1117–1119;
- 7fY. J. Cao, Y. Y. Lai, X. Wang, Y. J. Li, W. J. Xiao, Tetrahedron Lett. 2007, 48, 21–24.
- 8For reviews, see
- 8aM. T. Reetz, Proc. Natl. Acad. Sci. USA 2004, 101, 5716–5722;
- 8bC. M. Thomas, T. R. Ward, Chem. Soc. Rev. 2005, 34, 337–346;
- 8cY. Lu, Curr. Opin. Chem. Biol. 2005, 9, 118–126;
- 8dC. Letondor, T. R. Ward, ChemBioChem 2006, 7, 1845–1852;
- 8eR. Krämer, Angew. Chem. 2006, 118, 872–874;
10.1002/ange.200502907 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 858–860;
- 8fG. Roelfes, Mol. BioSyst. 2007, 3, 126–135.
- 9
- 9aM. E. Wilson, G. M. Whitesides, J. Am. Chem. Soc. 1978, 100, 306–307;
- 9bJ. Collot, J. Gradinaru, N. Humbert, M. Skander, A. Zocchi, T. R. Ward, J. Am. Chem. Soc. 2003, 125, 9030–9031;
- 9cJ. R. Carey, S. K. Ma, T. D. Pfister, D. K. Garner, H. K. Kim, J. A. Abramite, Z. Wang, Z. Guo and Y. Lu, J. Am. Chem. Soc. 2004, 126, 10812–10813;
- 9dM. Skander, N. Humbert, J. Collot, J. Gradinaru, G. Klein, A. Loosli, J. Sauser, A. Zocchi, F. Gilardoni, T. R. Ward, J. Am. Chem. Soc. 2004, 126, 14411–14418;
- 9eC. Letondor, N. Humbert, T. R. Ward, Proc. Natl. Acad. Sci. USA 2005, 102, 4683–4687;
- 9fT. Ueno, T. Koshiyama, M. Ohashi, K. Kondo, M. Kono, A. Suzuki, T. Yamane, Y. Watanabe, J. Am. Chem. Soc. 2005, 127, 6556–6562;
- 9gA. Mahammed, Z. Gross, J. Am. Chem. Soc. 2005, 127, 2883–2887;
- 9hH. Yamaguchi, T. Hirano, H. Kiminami, D. Taura, A. Harada, Org. Biomol. Chem. 2006, 4, 3571–3573;
- 9iM. T. Reetz, J. J.-P. Peyralans, A. Maichele, Y. Fu, M. Maywald, Chem. Commun. 2006, 4318–4320;
- 9jM. T. Reetz, N. Jiao, Angew. Chem. 2006, 118, 2476–2479;
10.1002/ange.200504561 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2416–2419.
- 10
- 10aG. Roelfes, B. L. Feringa, Angew. Chem. 2005, 117, 3294–3296;
10.1002/ange.200500298 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3230–3232;
- 10bG. Roelfes, A. J. Boersma, B. Feringa, Chem. Commun. 2006, 635–637;
- 10cA. J. Boersma, B. L. Feringa, G. Roelfes, Org. Lett. 2007, 9, 3647–3650.
- 11Recently, catalytic asymmetric fluorination reactions based on this concept were reported, which gave enantioselectivities up to 70 % ee: N. Shibata, H. Yasui, S. Nakamura, T. Toru, Synlett 2007, 1153–1157.
- 12For alternative approaches to DNA-based catalysis, see
- 12aM. Caprioara, R. Fiammengo, M. Engeser, A. Jäschke, Chem. Eur. J. 2007, 13, 2089–2095;
- 12bL. Ropartz, N. J. Meeuwenoord, G. A. van der Marel, P. W. N. M. van Leeuwen, A. M. Z. Slawin, P. C. J. Kamer, Chem. Commun. 2007, 1556–1558.
- 13
- 13aD. A. Evans, K. R. Fandrick, H. J. Song, J. Am. Chem. Soc. 2005, 127, 8942–8943;
- 13bD. A. Evans, H. J. Song, K. R. Fandrick, Org. Lett. 2006, 8, 3351–3354;
- 13cM. C. Myers, A. R. Bharadwaj, B. C. Milgram, K. A. Scheidt, J. Am. Chem. Soc. 2005, 127, 14675–14680.
- 14See the Supporting Information.
- 15R. P. Bell, The proton in chemistry, Ithica, Cornell University Press, 1959.
- 16For the pKa value of dialkyl malonic esters, see J. March, Advanced Organic Chemistry, 4th ed., Wiley, New York, 1992, p. 251.
- 17For R=Me, a small amount of an unidentified side product was detected (<8 %).
- 18S. Brandau, A. Landa, J. Franzen, M. Marigo, K. A. Jørgensen, Angew. Chem. 2006, 118, 4411–4415;
10.1002/ange.200601025 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4305–4309.
- 19F. Felluga, V. Gombac, G. Pitacco, E. Valentin, Tetrahedron: Asymmetry 2005, 16, 1341–1345.