Concise Enantio- and Diastereoselective Total Syntheses of Fumagillol, RK-805, FR65814, Ovalicin, and 5-Demethylovalicin†
Junichiro Yamaguchi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMaya Toyoshima
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorHideaki Kakeya Dr.
Antibiotics Laboratory, Discovery Research Institute, RIKEN, Wako, Saitama 351-0198, Japan
Search for more papers by this authorHiroyuki Osada Prof. Dr.
Antibiotics Laboratory, Discovery Research Institute, RIKEN, Wako, Saitama 351-0198, Japan
Search for more papers by this authorYujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorJunichiro Yamaguchi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMaya Toyoshima
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorHideaki Kakeya Dr.
Antibiotics Laboratory, Discovery Research Institute, RIKEN, Wako, Saitama 351-0198, Japan
Search for more papers by this authorHiroyuki Osada Prof. Dr.
Antibiotics Laboratory, Discovery Research Institute, RIKEN, Wako, Saitama 351-0198, Japan
Search for more papers by this authorYujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorThis paper is dedicated to Prof. E. J. Corey for his elegant total syntheses of the fumagillin and ovalicin families. This work was partially supported by a Grant-in-Aid for Scientific Research on Priority Areas 16073219 from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT).
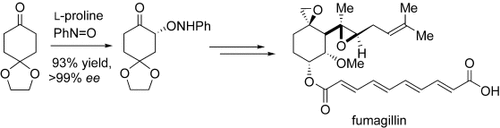
Graphical Abstract
L-Proline-mediated α-aminoxylation is a key step in the enantio- and diastereoselective total syntheses of fumagillin, ovalicin, and related compounds (see scheme). These compounds contain a cyclohexane ring, two epoxides, and five or six contiguous stereogenic centers, and they display anti-angiogenesis or immunosuppressive properties.
References
- 1
- 1aJ. Folkman, J. Natl. Cancer Inst. 1990, 82, 4;
- 1bW. Risau, Nature 1997, 386, 671;
- 1cM. Klagsbrun, M. A. Moses, Chem. Biol. 1999, 6, R 217;
- 1dG. Gasparini, Drugs 1999, 58, 17.
- 2
- 2aH. Kakeya, R. Onose, H. Koshino, A. Yoshida, K. Kobayashi, S.-I. Kageyama, H. Osada, J. Am. Chem. Soc. 2002, 124, 3496;
- 2bH. Kakeya, R. Onose, A. Yoshida, H. Koshino, H. Osada, J. Antibiot. 2002, 55, 829;
- 2cM. Shoji, J. Yamaguchi, H. Kakeya, H. Osada, Y. Hayashi, Angew. Chem. 2002, 114, 3324;
Angew. Chem. Int. Ed. 2002, 41, 3192;
10.1002/1521-3773(20020902)41:17<3192::AID-ANIE3192>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 2dM. Shoji, S. Kishida, M. Takeda, H. Kakeya, H. Osada, Y. Hayashi, Tetrahedron Lett. 2002, 43, 9155;
- 2eM. Shoji, H. Imai, I. Shiina, H. Kakeya, H. Osada, Y. Hayashi, J. Org. Chem. 2004, 69, 1548.
- 3
- 3aY. Asami, H. Kakeya, R. Onose, A. Yoshida, H. Matsuzaki, H. Osada, Org. Lett. 2002, 4, 2845;
- 3bY. Hayashi, M. Shoji, J. Yamaguchi, K. Sato, S. Yamaguchi, T. Mukaiyama, K. Sakai, Y. Asami, H. Kakeya, H. Osada, J. Am. Chem. Soc. 2002, 124, 12078.
- 4Y. Asami, H. Kakeya, R. Onose, Y.-H. Chang, M. Toi, H. Osada, Tetrahedron 2004, 60, 7085.
- 5M. C. McCowen, M. E. Callender, J. F. Lawlis, Science 1951, 113, 202.
- 6D. Ingber, T. Fujita, S. Kishimoto, K. Sudo, T. Kanamaru, H. Brem, J. Folkman, Nature 1990, 348, 555.
- 7H. Sigg, H. P. Weber, Helv. Chim. Acta 1968, 51, 1395.
- 8K.-H. Son, J.-Y. Kwon, H.-W. Jeong, H.-K. Kim, C.-J. Kim, Y.-H. Chang, J.-D. Choi, B.-M. Kwon, Bioorg. Med. Chem. 2002, 10, 185.
- 9H. Hatanaka, T. Kino, M. Hashimoto, Y. Tsurumi, A. Kuroda, H. Tanaka, T. Goto, M. Okuhara, J. Antibiot. 1988, 41, 999.
- 10
- 10aG. Zhou, C. W. Tsai, J. O. Liu, J. Med. Chem. 2003, 46, 3452;
- 10bV. Rodeschini, J.-G. Boiteau, P. V. de Weghe, C. Tarnus, J. Eustache, J. Org. Chem. 2004, 69, 357;
- 10cR. Maztischek, A. Huwe, A. Giannis, Org. Biomol. Chem. 2005, 3, 2150, and references therein.
- 11
- 11aE. J. Corey, B. B. Snider, J. Am. Chem. Soc. 1972, 94, 2549;
- 11bE. J. Corey, J. P. Dittami, J. Am. Chem. Soc. 1985, 107, 256;
- 11cD. A. Vosburg, S. Weiler, E. J. Sorensen, Angew. Chem. 1999, 111, 1022;
10.1002/(SICI)1521-3757(19990401)111:7<1024::AID-ANGE1024>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 1999, 38, 971;10.1002/(SICI)1521-3773(19990401)38:7<971::AID-ANIE971>3.0.CO;2-W PubMed Web of Science® Google Scholar
- 11dM. Hutchings, D. Moffat, N. S. Simpkins, Synlett 2001, 661.
- 12A. Barco, S. Benetti, C. D. Risi, P. Marchetti, G. P. Pollini, V. Zanirato, Tetrahedron: Asymmetry 1998, 9, 2857.
- 13
- 13aS. Bath, D. C. Billington, S. D. Gero, B. Quiclet-Sire, M. Samadi, J. Chem. Soc. Chem. Commun. 1994, 1495;
- 13bD. H. R. Barton, S. Bath, D. C. Billington, S. D. Gero, B. Quiclet-Sire, M. Samadi, J. Chem. Soc. Perkin Trans. 1 1995, 1551.
- 14D. F. Taber, T. E. Christos, A. L. Rheingold, I. A. Guzei, J. Am. Chem. Soc. 1999, 121, 5589.
- 15D. Kim, S. K. Ahn, H. Bae, W. J. Choi, H. S. Kim, Tetrahedron Lett. 1997, 38, 4437.
- 16O. Bedel, A. Haudrechy, Y. Langlois, Eur. J. Org. Chem. 2004, 3813.
- 17
- 17aS. Amano, N. Ogawa, M. Ohtsuka, S. Ogawa, N. Chida, Chem. Commun. 1998, 1263;
- 17bS. Amano, N. Ogawa, M. Ohtsuka, N. Chida, Tetrahedron 1999, 55, 2205.
- 18D. A. Vosburg, S. Weiler, E. J. Sorensen, Chirality 2003, 15, 156.
- 19J.-G. Boiteau, P. Van de Weghe, J. Eustache, Org. Lett. 2001, 3, 2737.
- 20E. J. Corey, A. Guzman-Perez, M. C. Noe, J. Am. Chem. Soc. 1994, 116, 12109.
- 21
- 21aY. Hayashi, J. Yamaguchi, K. Hibino, M. Shoji, Tetrahedron Lett. 2003, 44, 8293;
- 21bY. Hayashi, J. Yamaguchi, T. Sumiya, M. Shoji, Angew. Chem. 2004, 116, 1132; Angew. Chem. Int. Ed. 2004, 43, 1112;
- 21cY. Hayashi, J. Yamaguchi, T. Sumiya, K. Hibino, M. Shoji, J. Org. Chem. 2004, 69, 5966;
- 21dY. Hayashi, J. Yamaguchi, K. Hibino, T. Sumiya, T. Urushima, M. Shoji, D. Hashizume, H. Koshino, Adv. Synth. Catal. 2004, 346, 1435;
- 21eG. Zhong, Angew. Chem. 2003, 115, 4379; Angew. Chem. Int. Ed. 2003, 42, 4247;
- 21fS. P. Brown, M. P. Brochu, C. J. Sinz, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 10808;
- 21gA. Bøgevig, H. Sundeen, A. Córdova, Angew. Chem. 2004, 116, 1129;
10.1002/ange.200353018 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1109;
- 21hA. Córdova, H. Sunden, A. Bøgevig, M. Johansson, F. Himo, Chem. Eur. J. 2004, 10, 3673;
- 21iN. Momiyama, H. Torii, S. Saito, H. Yamamoto, Proc. Natl. Acad. Sci. USA 2004, 101, 5374;
- 21jW. Wang, J. Wang, H. Li, L. Liao, Tetrahedron Lett. 2004, 45, 7235;
- 21kH. Sunden, N. Dahlin, I. Ibrahem, H. Adolfsson, A. Córdova, Tetrahedron Lett. 2005, 46, 3385; for a review, see:
- 21lP. Merino, T. Tejero, Angew. Chem. 2004, 116, 3055; Angew. Chem. Int. Ed. 2004, 43, 2995.
- 22E. J. Corey, M. Chaykovsky, J. Am. Chem. Soc. 1965, 87, 1353.
- 23K. B. Sharpless, R. C. Michaelson, J. Am. Chem. Soc. 1973, 95, 6136.
- 24S. Kobayashi, Y. Tsuchiya, T. Mukaiyama, Chem. Lett. 1991, 537.
- 25For hydroxy-directed Michael reaction, see:
- 25aK. A. Swiss, D. C. Liotta, C. A. Maryanoff, J. Am. Chem. Soc. 1990, 112, 9393;
- 25bK. A. Swiss, W. Hinkley, C. A. Maryanoff, D. C. Liotta, Synthesis 1992, 127;
- 25cM. Solomon, W. C. L. Jamison, M. MaCormick, D. Liotta, D. A. Cherry, J. E. Mills, R. D. Shah, J. D. Rodgers, C. A. Maryanoff, J. Am. Chem. Soc. 1988, 110, 3702.
- 26For Michael reactions of zincate, see:
- 26aM. Suzuki, Y. Morita, H. Koyano, M. Koga, R. Noyori, Tetrahedron 1990, 46, 4809;
- 26bA. Fürstner, K. Grela, C. Mathes, C. W. Lehmann, J. Am. Chem. Soc. 2000, 122, 11799.
- 27Vinyl bromide 14 was prepared by Sorensen's modified route[11c,18] of a synthetic procedure originally reported by Corey et al.; see: E. J. Corey, J. Lee, B. E. Roberts, Tetrahedron Lett. 1997, 38, 8915.
- 28G. M. Rubottom, M. A. Vazquez, D. R. Pellagrina, Tetrahedron Lett. 1974, 4319.
- 29When the spiro epoxide is formed first, the diastereoselectivity of the epoxidation of the side chain is not high. See also reference [11c].
- 30
- 30aD. B. Dess, J. C. Martin, J. Org. Chem. 1983, 48, 4155;
- 30bD. B. Dess, J. C. Martin, J. Am. Chem. Soc. 1991, 113, 7277;
- 30cR. E. Ireland, L. Liu, J. Org. Chem. 1993, 58, 2899.
- 31Epoxidation of the side chain was performed as the last step of Corey's synthesis;[11b] however, we thought it was better to oxidize much earlier owing to the instability of the precursor.
- 32D. R. Pesiri, D. K. Morita, T. Walker, W. Tumas, Organometallics 1990, 18, 4916.
- 33T. W. Greene, P. G. M. Wuts, Protective Groups in Organic Synthesis, 3rd ed., Wiley, New York, 1999.
10.1002/0471220574 Google Scholar
- 34The stereochemistry of 28 and 29 was not determined.
- 35D. E. Cane, R. H. Levin, J. Am. Chem. Soc. 1975, 97, 1282.