Mechanism of 4,6-O-Benzylidene-Directed β-Mannosylation as Determined by α-Deuterium Kinetic Isotope Effects†
David Crich Prof. Dr.
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607-7061, USA, Fax: (+1) 312-996-4439
Search for more papers by this authorN. Susantha Chandrasekera
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607-7061, USA, Fax: (+1) 312-996-4439
Search for more papers by this authorDavid Crich Prof. Dr.
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607-7061, USA, Fax: (+1) 312-996-4439
Search for more papers by this authorN. Susantha Chandrasekera
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, IL 60607-7061, USA, Fax: (+1) 312-996-4439
Search for more papers by this authorWe thank the National Institutes of Health (GM 62160) for support of our work in this area
Graphical Abstract
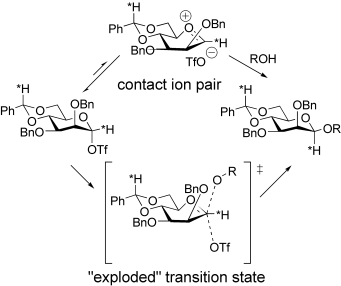
Considerable oxacarbenium ion character may be in the transition state of a highly β-selective mannosylation reaction that proceeds via an α-mannosyl triflate. An α-deuterium kinetic isotope effect of 1.2 was measured at −78 °C (≡1.1 at 25 °C). This information may be interpreted in terms of a stereoselective trapping of a transient contact ion pair or, alternatively, as representative of an “exploded” transition state (see scheme).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53688_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. A. Dwek, T. D. Butters, Chem. Rev. 2002, 102, 283.
- 2 Comprehensive Natural Products Chemistry, Vol. 3 (Eds.: ), Pergamon, Oxford, 1999.
- 3 Carbohydrates in Chemistry and Biology, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 2000.
- 4In 1994 >700 glycosides were synthesized chemically using hundreds of combinations of donors and promoters: F. Barresi, O. Hindsgaul, J. Carbohydr. Chem. 1995, 14, 1043.
- 5R. U. Lemieux, K. B. Hendriks, R. V. Stick, K. James, J. Am. Chem. Soc. 1975, 97, 4056.
- 6For example see:
- 6aB. Capon, Chem. Rev. 1969, 69, 407;
- 6bA. J. Rhind-Tutt, C. A. Vernon, J. Chem. Soc. 1960, 4637;
- 6cB. Capon, P. M. Collins, A. A. Levy, W. G. Overend, J. Chem. Soc. 1964, 3242;
- 6dL. R. Schroeder, J. W. Green, D. C. Johnson, J. Chem. Soc. B 1966, 447.
- 7
- 7aJ. E. Wallace, L. R. Schroeder, J. Chem. Soc. Perkin Trans. 2 1977, 795;
- 7bJ. E. Wallace, L. R. Schroeder, J. Chem. Soc. Perkin Trans. 2 1976, 1632.
- 8In contrast there are numerous quantitative and semiquantitative studies on substituent effects on glycosylation rates:
- 8aN. L. Douglas, S. V. Ley, U. Lucking, S. L. Warriner, J. Chem. Soc. Perkin Trans. 1 1998, 51;
- 8bZ. Zhang, I. R. Ollmann, X.-S. Ye, R. Wischnat, T. Baasov, C.-H. Wong, J. Am. Chem. Soc. 1999, 121, 734;
- 8cC. P. J. Glaudemans, H. G. Fletcher, J. Am. Chem. Soc. 1965, 87, 4636;
- 8dL. G. Green, S. V. Ley, in Carbohydrates in Chemistry and Biology, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 2000, p. 427.
10.1002/9783527618255.ch17 Google Scholar
- 9
- 9aG. J. Davies, M. L. Sinnott, S. G. Withers in Comprehensive Biological Catalysis, Vol. 1 (Ed.: ), Academic Press, London, 1998, p. 119;
- 9bB. W. Murray, V. Wittmann, M. D. Burkart, S.-C. Hung, C.-H. Wong, Biochemistry 1997, 36, 823;
- 9cY. Tanaka, W. Tao, J. S. Blanchard, E. J. Hehre, J. Biol. Chem. 1994, 269, 32306;
- 9dA. M. MacLeod, D. Tull, K. Rupitz, A. J. Warren, S. G. Withers, Biochemistry 1996, 35, 13165;
- 9eD. L. Zechel, S. G. Withers, Acc. Chem. Res. 2000, 33, 11.
- 10
- 10aD. Crich, S. Sun, J. Org. Chem. 1997, 62, 1198;
- 10bD. Crich, S. Sun, Tetrahedron 1998, 54, 8321;
- 10cD. Crich in Glycochemistry: Principles, Synthesis, and Applications, (Eds.: ), Marcel Dekker, New York, 2001, p. 53.
10.1201/9780585407500.ch3 Google Scholar
- 11D. Crich, S. Sun, J. Am. Chem. Soc. 1997, 119, 11217.
- 12D. Crich, M. Smith, J. Am. Chem. Soc. 2001, 123, 9015.
- 13 Reaction Rates of Isotopic Molecules (Eds.: ), Wiley-Interscience, New York, 1980.
- 14Displacement of triflate from preformed glycosyl triflates is complete in minutes −78 °C,[11] ruling out the use of kinetic measurements, at least by the NMR methods used to characterize these intermediates.
- 15On grounds of the amount of substrate needed, the substrate solubility, and the instrument time needed, direct determination of kinetic isotope effects by NMR spectroscopy at natural abundance (as in
- 15aD. A. Singleton, A. A. Thomas, J . Am. Chem. Soc. 1995, 117, 9357;
- 15bD. A. Singleton, M. J. Szymanski, J. Am. Chem. Soc. 1999, 121, 9455;
- 15cJ. K. Lee, A. D. Bain, P. J. Berti, J. Am. Chem. Soc. 2004, 126, 3769) was deemed impractical.
- 16The critical feature is the liberation of the deuteriated mannose from 2 with neopentyl glycol and PTSA which minimizes isomerization to glucose.
- 172H NMR spectroscopy was investigated but the linewidths and chemical shifts were such that the integration was compromised.
- 18D. Crich, M. Smith, Q. Yao, J. Picione, Synthesis 2001, 323.
- 19Compound 8, a more soluble version of 1-benzenesulfinyl piperidine,[12] permits activation at −78 °C rather than the −60 °C needed for the latter.
- 20An excess of 8 and Tf2O was employed to ensure complete conversion of 6 to 9, as verified on the NMR spectrum of the crude reaction mixture following glycosylation, such that any isotope effects observed do not result from the initial activation step.
- 21P. J. Garegg, H. Hultberg, S. Wallin, Carbohydr. Res. 1982, 108, 97.
- 22The yield of 12 was <5 %, ruling out determination of the KIE for its formation, but allowing it to be neglected in the calculation of the KIE of 11.
- 23Ideally, comparison of the KIEs on the α- and β-products in an unselective coupling would be informative. However, this is very difficult to achieve as the method employed requires baseline resolution of the various anomeric and benzylidene acetal signals. In practice, this has proven to be a limitation with numerous substrates assayed, particularly for unselective reactions when the number of similar signals is multiplied. Second, while the reaction conditions can be manipulated[10a,b] to give predominantly the α-product it is doubtful whether measurements made under such circumstances are relevant as they presumably do not proceed via the intermediate glycosyl triflate.
- 24Assuming T(ln kH/kD) is constant and no tunnelling occurs.
- 25
- 25aA. J. Bennet, M. L. Sinnott, J. Am. Chem. Soc. 1986, 108, 7287;
- 25bD. Indurugalla, A. J. Bennet, J. Am. Chem. Soc. 2001, 123, 10889;
- 25cA. J. Bennet, M. L. Sinnott, W. S. S. Wijesundera, J. Chem. Soc. Perkin Trans. 2 1985, 1233.
- 26 Y. Zhang, J. Bommuswamy, M. L. Sinnott, J. Am. Chem. Soc. 1994, 116, 7557.
- 27The distinction between the two mechanisms is a fine one and cannot be made on the basis of the available data. Exactly analogous problems arise in the study of the hydrolysis of simple glycosides,[25] in that of glycosyl fluorides,[26] and in enzymic glycoside hydrolysis and glycosyl transfer.[9] Comparisons with KIEs determined for enzymic reactions must, however, be treated with caution since the enzyme likely binds the substrate in a nonstandard conformation. For example: G. Sulzenbacher, H. Driguez, B. Henrissat, M. Schulein, G. J. Davies, Biochemistry 1996, 35, 15280.
- 28C. W. Andrews, R. Rodebaugh, B. Fraser-Reid, J. Org. Chem. 1996, 61, 5280.
- 29Alternative explanations:
- 29aT. Nukada, A. Berces, D. M. Whitfield, Carbohydr. Res. 2002, 337, 765;
- 29bH. H. Jensen, L. U. Nordstrøm, M. Bols, J. Am. Chem. Soc. 2004, 126, 9205.
- 30
- 30aG. A. Olah, D. G. Parker, N. Yoneda, J. Org. Chem. 1977, 42, 32;
- 30bD. A. Forsyth, V. A. Osterman, J. R. DeMember, J. Am. Chem. Soc. 1985, 107, 818.
- 31
- 31aT. L. Amyes, W. P. Jencks, J. Am. Chem. Soc. 1989, 111, 7888;
- 31bM. N. Namchuk, J. D. McCarter, A. Becalski, T. Andrews, S. G. Withers, J. Am. Chem. Soc. 2000, 122, 1270;
- 31cJ. Zhu, A. J. Bennet, J. Am. Chem. Soc. 1998, 120, 3887.
- 32The fact that the covalent α-triflate 9 is so heavily favored argues against the possibility that the effects measured here result from an equilibrium isotope effect. Likewise, the highly stereoselective nature of the coupling argues against the effects measured arising from any significant shift in the equilibrium position as this would necessarily be associated with reduced selectivity.
- 33On the grounds that the reaction studied is highly stereoselective, and alcohol 10 is a typical carbohydrate the results obtained here may reasonably be considered as representative of this class of reactions. Of course exceptions may exist, particularly with extremely selective cases using highly reactive alcohols, but they are not likely to be representative of the formation of true interglycosidic bonds.