Conjugated Dienyne-Imides as Robust Precursors of 1-Azatrienes for 6π Electrocyclizations to Furo[2,3-b]dihydropyridine Cores
Yanjun Wan
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorDr. Xuchun Zheng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Cheng Ma
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYanjun Wan
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorDr. Xuchun Zheng
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Cheng Ma
Department of Chemistry, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorAbstract
A novel strategy to generate functionalized 1-azatriene intermediates for 6π electrocyclizations was developed by using readily accessible dienyne-imides and various terminal olefins under PdII catalysis. Taking advantage of the sequential cooperation between preloaded and incorporated functional handles at 1,3-dien-5-yne skeletons, this method not only enables the selective generation of putative 1-azatrienes but significantly accelerates their thermal 6π-electrocyclic ring-closure processes to a series of highly substituted furo[2,3-b]dihydropyridine derivatives in good yields.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange201800303-sup-0001-misc_information.pdf6.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aS. N. Sirakanyan, A. A. Hovakimyan, A. S. Noravyan, Russ. Chem. Rev. 2015, 84, 441;
- 1bA. Jasselin-Hinschberger, C. Comoy, A. Chartoire, Y. Fort, Eur. J. Org. Chem. 2015, 2321.
- 2
- 2aA. L. Rodriguez, R. Williams, Y. Zhou, S. R. Lindsley, U. Le, M. D. Grier, C. D. Weaver, P. J. Conn, C. W. Lindsley, Bioorg. Med. Chem. Lett. 2009, 19, 3209;
- 2bJ. S. Debenham, C. B. Madsen-Duggan, R. B. Toupence, T. F. Walsh, J. Wang, X. Tong, S. Kumar, J. Lao, T. M. Fong, J. C. Xiao, C. R. R. C. Huang, C.-P. Shen, Y. Feng, D. J. Marsh, D. S. Stribling, L. P. Shearman, A. M. Strack, M. T. Goulet, Bioorg. Med. Chem. Lett. 2010, 20, 1448;
- 2cK. Parcella, K. Eastman, K.-S. Yeung, K. A. Grant-Young, J. Zhu, T. Wang, Z. Zhang, Z. Yin, D. Parker, K. Mosure, H. Fang, Y.-K. Wang, J. Lemm, X. Zhuo, U. Hanumegowda, M. Liu, K. Rigat, M. Donoso, M. Tuttle, T. Zvyaga, Z. Haarhoff, N. A. Meanwell, M. G. Soars, S. B. Roberts, J. F. Kadow, ACS Med. Chem. Lett. 2017, 8, 771.
- 3Selected examples:
- 3aS. Shiotani, Heterocycles 1997, 45, 975;
- 3bA. Arcadi, S. Cacchi, S. Di Giuseppe, G. Fabrizi, F. Marinelli, Org. Lett. 2002, 4, 2409;
- 3cE. Bossharth, P. Desbordes, N. Monteiro, G. Balme, Org. Lett. 2003, 5, 2441;
- 3dA. Fayol, J. Zhu, Org. Lett. 2004, 6, 115;
- 3eG. L. Beutner, J. T. Kuethe, Y. Nobuyoshi, Tetrahedron Lett. 2009, 50, 781;
- 3fN. M. Barl, E. Sansiaume-Dagousset, G. Monzón, A. J. Wagner, P. Knochel, Org. Lett. 2014, 16, 2422;
- 3gF. Fumagalli, F. da Silva Emery, J. Org. Chem. 2016, 81, 10339.
- 4An elegant review of biosynthetic and biomimetic electrocyclizations: C. M. Beaudry, J. P. Malerich, D. Trauner, Chem. Rev. 2005, 105, 4757.
- 5A review:
- 5aK. Tanaka, K. Fukase, S. Katsumura, Synlett 2011, 2115. For selected examples:
- 5bP. Schiess, H. L. Chia, P. Ringele, Tetrahedron Lett. 1972, 13, 313;
10.1016/S0040-4039(01)84311-2 Google Scholar
- 5cA. R. de Lera, W. Reischl, W. H. Okamura, J. Am. Chem. Soc. 1989, 111, 4051;
- 5dD. F. Maynard, W. H. Okamura, J. Org. Chem. 1995, 60, 1763;
- 5eK. Tanaka, H. Mori, M. Yamamoto, S. Katsumura, J. Org. Chem. 2001, 66, 3099;
- 5fK. Tanaka, S. Katsumura, J. Am. Chem. Soc. 2002, 124, 9660;
- 5gM. L. Meketa, S. M. Weinreb, Y. Nakao, N. Fusetani, J. Org. Chem. 2007, 72, 4892;
- 5hB. W.-Q. Hui, S. Chiba, Org. Lett. 2009, 11, 729;
- 5iM. Alajarin, B. Bonillo, M.-M. Ortin, P. Sanchez-Andrada, A. Vidal, Eur. J. Org. Chem. 2011, 1896;
- 5jX. Liu, N. Zhang, J. Yang, Y. Liang, R. Zhang, D. Dong, J. Org. Chem. 2013, 78, 3323;
- 5kY. Jiang, C.-M. Park, T.-P. Loh, Org. Lett. 2014, 16, 3432;
- 5lB. M. Trost, B. Biannic, Org. Lett. 2015, 17, 1433.
- 6
- 6aH. M. Sklenicka, R. P. Hsung, M. J. McLaughlin, L.-L. Wei, A. I. Gerasyuto, W. W. Brennessel, J. Am. Chem. Soc. 2002, 124, 10435;
- 6bN. Sydorenko, R. P. Hsung, E. L. Vera, Org. Lett. 2006, 8, 2611;
- 6cB. M. Trost, A. C. Gutierrez, Org. Lett. 2007, 9, 1473;
- 6dD. A. Colby, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2008, 130, 3645;
- 6eA. Patel, G. A. Barcan, O. Kwon, K. N. Houk, J. Am. Chem. Soc. 2013, 135, 4878;
- 6fZ.-X. Ma, A. Patel, K. N. Houk, R. P. Hsung, Org. Lett. 2015, 17, 2138;
- 6gH. Wei, Y. Li, K. Xiao, B. Cheng, H. Wang, L. Hu, H. Zhai, Org. Lett. 2015, 17, 5974;
- 6hB. J. Fallon, J.-B. Garsi, E. Derat, M. Amatore, C. Aubert, M. Petit, ACS Catal. 2015, 5, 7493;
- 6iE. M. Phillips, T. Mesganaw, A. Patel, S. Duttwyler, B. Q. Mercado, K. N. Houk, J. A. Ellman, Angew. Chem. Int. Ed. 2015, 54, 12044; Angew. Chem. 2015, 127, 12212.
- 7Reviews:
- 7aD. M. Hitt, J. M. O'Connor, Chem. Rev. 2011, 111, 7904;
- 7bG. Zimmermann, Eur. J. Org. Chem. 2001, 457. For leading examples:
- 7cH. Hopf, H. Musso, Angew. Chem. Int. Ed. Engl. 1969, 8, 680; Angew. Chem. 1969, 81, 704;
- 7dL. Kaplan, S. P. Walch, K. E. Wilzbach, J. Am. Chem. Soc. 1968, 90, 5646.
- 8Selected reviews on transition-metal-catalyzed transformations of polyenynes toward heterocycles:
- 8aC. Raviola, S. Protti, D. Ravelli, M. Fagnoni, Chem. Soc. Rev. 2016, 45, 4364;
- 8bA. V. Gulevich, A. S. Dudnik, N. Chernyak, V. Gevorgyan, Chem. Rev. 2013, 113, 3084;
- 8cY.-Q. Deng, A. K. Å. Persson, J.-E. Bäckvall, Chem. Eur. J. 2012, 18, 11498. A comprehensive review on the diverse reactivity of 1,3-dien-5-ynes:
- 8dE. Aguilar, R. Sanz, M. A. Fernández-Rodríguez, P. García-García, Chem. Rev. 2016, 116, 8256.
- 9Selected examples:
- 9aC. A. Merlic, M. E. Pauly, J. Am. Chem. Soc. 1996, 118, 11319;
- 9bK. Maeyama, N. Iwasawa, J. Am. Chem. Soc. 1998, 120, 1928;
- 9cJ. W. Dankwardt, Tetrahedron Lett. 2001, 42, 5809;
- 9dH.-C. Shen, S. Pal, J.-J. Lian, R.-S. Liu, J. Am. Chem. Soc. 2003, 125, 15762;
- 9eJ. Barluenga, M. A. Fernández-Rodríguez, P. García-García, E. Aguilar, J. Am. Chem. Soc. 2008, 130, 2764;
- 9fC. Hulot, G. Blond, J. Suffert, J. Am. Chem. Soc. 2008, 130, 5046;
- 9gA. K. Basak, M. A. Tius, Org. Lett. 2008, 10, 4073;
- 9hP. García-García, M. A. Fernández-Rodríguez, E. Aguilar, Angew. Chem. Int. Ed. 2009, 48, 5534; Angew. Chem. 2009, 121, 5642;
- 9iA. Martínez, P. García-García, M. A. Fernández-Rodríguez, F. Rodríguez, R. Sanz, Angew. Chem. Int. Ed. 2010, 49, 4633; Angew. Chem. 2010, 122, 4737;
- 9jD. Vasu, H.-H. Hung, S. Bhunia, S. A. Gawade, A. Das, R.-S. Liu, Angew. Chem. Int. Ed. 2011, 50, 6911; Angew. Chem. 2011, 123, 7043;
- 9kT. Shibata, S. Takayasu, S. Yuzawa, T. Otani, Org. Lett. 2012, 14, 5106;
- 9lJ. Sheng, C. Fan, Y. Ding, X. Fan, J. Wu, Chem. Commun. 2014, 50, 4188.
- 10Reviews:
- 10aY. Yamamoto, J. Org. Chem. 2007, 72, 7817;
- 10bI. V. Alabugin, K. Gilmore, Chem. Commun. 2013, 49, 11246;
- 10cJ. W. Herndon, Coord. Chem. Rev. 2018, 356, 1.
- 11
- 11aH. Tsuchikawa, Y. Maekawa, S. Katsumura, Org. Lett. 2012, 14, 2326;
- 11bY. Maekawa, T. Sakaguchi, H. Tsuchikawa, S. Katsumura, Tetrahedron Lett. 2012, 53, 837.
- 12Reviews of cross-conjugated systems:
- 12aN. F. Phelan, M. Orchin, J. Chem. Educ. 1968, 45, 633;
- 12bM. Gholami, R. R. Tykwinski, Chem. Rev. 2006, 106, 4997. For a recent review on the transition-metal-catalyzed synthesis of conjugated enynes, see:
- 12cY. Zhou, Y. Zhang, J. Wang, Org. Biomol. Chem. 2016, 14, 6638.
- 13
- 13aD. Cheng, F. Ling, Z. Li, W. Yao, C. Ma, Org. Lett. 2012, 14, 3146;
- 13bZ. Li, F. Ling, D. Cheng, C. Ma, Org. Lett. 2014, 16, 1822;
- 13cF. Ling, Z. Li, C. Zheng, X. Liu, C. Ma, J. Am. Chem. Soc. 2014, 136, 10914;
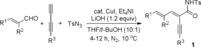
- 13dJ. Wu, D. Wang, Y. Wan, C. Ma, Chem. Commun. 2016, 52, 1661. Dienyne-imides 1 were synthesized from corresponding acrylaldehydes, 1,3-butadiynes, and tosyl azide in gram scale as following (see the Supporting Information):
- 14CCDC 1814857, 1814858, 1814859, 1814860, and 1814861 (1 l, 3 ga, 4 ab, 5 ab, and 3 kh-Pd) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 15
- 15aM. Catellani, F. Frignani, A. Rangoni, Angew. Chem. Int. Ed. Engl. 1997, 36, 119; Angew. Chem. 1997, 109, 142;
- 15bN. Della Cá, M. Fontana, E. Motti, M. Catellani, Acc. Chem. Res. 2016, 49, 1389.
- 16A discussion on kinetics of thermal electrocyclic ring closure:
- 16aC. W. Spangler, T. P. Jondahl, B. Spangler, J. Org. Chem. 1975, 58, 2478. For a review of “Rollover” cyclometalation:
- 16bB. Butschke, H. Schwarz, Chem. Sci. 2012, 3, 308.
- 17A. T. Balaban, D. C. Oniciu, A. R. Katritzky, Chem. Rev. 2004, 104, 2777.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




