A Robust Nickel Catalyst for Cyanomethylation of Aldehydes: Activation of Acetonitrile under Base-Free Conditions†
Dr. Sumit Chakraborty
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorYogi J. Patel
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorDr. Jeanette A. Krause
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorCorresponding Author
Prof. Hairong Guan
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.htmlSearch for more papers by this authorDr. Sumit Chakraborty
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorYogi J. Patel
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorDr. Jeanette A. Krause
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Search for more papers by this authorCorresponding Author
Prof. Hairong Guan
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.html
Department of Chemistry, University of Cincinnati, P. O. Box 210172, Cincinnati, OH 45221-0172 (USA) http://homepages.uc.edu/∼guanhg/index.htmlSearch for more papers by this authorWe thank the U.S. National Science Foundation (CHE-0952083) for support of this research. X-Ray data were collected on a Bruker SMART6000 diffractometer which was purchased with funding provided by an NSF-MRI grant (CHE-0215950).
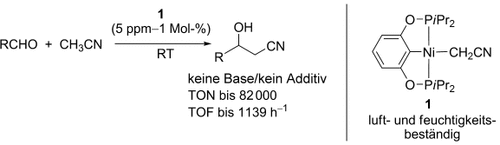
Graphical Abstract
Wer braucht schon Basen? Der Nickel-Cyanmethyl-Komplex 1 katalysiert die Kupplung von Aldehyden mit Acetonitril bei Raumtemperatur ohne Basenzusatz. Das langlebige und bemerkenswert effiziente Katalysatorsystem erreicht hohe Umsatzzahlen (TON) und -frequenzen (TOF), und die milden Reaktionsbedingungen ermöglichen den Einsatz auch basenempfindlicher Aldehyde.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201302613_sm_miscellaneous_information.pdf3.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Kamal, G. B. R. Khanna, R. Ramu, Tetrahedron: Asymmetry 2002, 13, 2039–2051;
- 1bY. Fukuda, Y. Okamoto, Tetrahedron 2002, 58, 2513–2521;
- 1cS. Kamila, D. Zhu, E. R. Biehl, L. Hua, Org. Lett. 2006, 8, 4429–4431;
- 1dH. Ankati, D. Zhu, Y. Yang, E. R. Biehl, L. Hua, J. Org. Chem. 2009, 74, 1658–1662.
- 2
- 2aE. M. Kaiser, C. R. Hauser, J. Am. Chem. Soc. 1967, 89, 4566–4567;
- 2bY. Lin, X. Zhu, M. Xiang, J. Organomet. Chem. 1993, 448, 215–218;
- 2cS.-I. Murahashi, T. Naota, H. Taki, M. Mizuno, H. Takaya, S. Komiya, Y. Mizuho, N. Oyasato, M. Hiraoka, M. Hirano, A. Fukuoka, J. Am. Chem. Soc. 1995, 117, 12436–12451;
- 2dR. Kuwano, H. Miyazaki, Y. Ito, Chem. Commun. 1998, 71–72;
- 2eF. F. Fleming, B. C. Shook, Tetrahedron 2002, 58, 1–23;
- 2fK. Motokura, D. Nishimura, K. Mori, T. Mizugaki, K. Ebitani, K. Kaneda, J. Am. Chem. Soc. 2004, 126, 5662–5663;
- 2gH. W. Cheung, J. Li, W. Zheng, Z. Zhou, Y. H. Chiu, Z. Lin, C. P. Lau, Dalton Trans. 2010, 39, 265–274;
- 2hR. Yazaki, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 5522–5531.
- 3
- 3aE. M. Kaiser, C. R. Hauser, J. Org. Chem. 1968, 33, 3402–3404;
- 3bN.-S. Li, S. Yu, G. W. Kabalka, J. Org. Chem. 1995, 60, 5973–5974;
- 3cP. Kisanga, D. McLeod, B. D’Sa, J. Verkade, J. Org. Chem. 1999, 64, 3090–3094;
- 3dA. L. Rodriguez, T. Bunlaksananusorn, P. Knochel, Org. Lett. 2000, 2, 3285–3287;
- 3eT. Bunlaksananusorn, A. L. Rodriguez, P. Knochel, Chem. Commun. 2001, 745–746;
- 3fR. Sott, J. Granander, G. Hilmersson, Chem. Eur. J. 2002, 8, 2081–2087;
10.1002/1521-3765(20020503)8:9<2081::AID-CHEM2081>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 3gR. Sott, J. Granander, G. Hilmersson, J. Am. Chem. Soc. 2004, 126, 6798–6805;
- 3hF. F. Fleming, S. Gudipati, Z. Zhang, W. Liu, O. W. Steward, J. Org. Chem. 2005, 70, 3845–3849;
- 3iF. F. Fleming, V. A. Vu, B. C. Shook, M. Rahman, O. W. Steward, J. Org. Chem. 2007, 72, 1431–1436;
- 3jF. F. Fleming, W. Liu, S. Ghosh, O. W. Steward, J. Org. Chem. 2008, 73, 2803–2810;
- 3kF. F. Fleming, S. Gudipati, Eur. J. Org. Chem. 2008, 5365–5374.
- 4
- 4aY. Kawano, N. Kaneko, T. Mukaiyama, Chem. Lett. 2005, 34, 1508–1509;
- 4bS. Matsukawa, E. Kitazaki, Tetrahedron Lett. 2008, 49, 2982–2984;
- 4cK. Wadhwa, J. G. Verkade, J. Org. Chem. 2009, 74, 5683–5686.
- 5
- 5aC. Palomo, J. M. Aizpurua, M. C. López, B. Lecea, J. Chem. Soc. Perkin Trans. 1 1989, 1692–1694;
- 5bR. Latouche, F. Texier-Boullet, J. Hamelin, Tetrahedron Lett. 1991, 32, 1179–1182;
- 5cY. Kawanami, H. Yuasa, F. Toriyama, S. Yoshida, T. Baba, Catal. Commun. 2003, 4, 455–459;
- 5dY. Suto, N. Kumagai, S. Matsunaga, M. Kanai, M. Shibasaki, Org. Lett. 2003, 5, 3147–3150.
- 6Y.-C. Fan, G.-F. Du, W.-F. Sun, W. Kang, L. He, Tetrahedron Lett. 2012, 53, 2231–2233.
- 7
- 7aN. Kumagai, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 13632–13633;
- 7bN. Kumagai, S. Matsunaga, M. Shibasaki, Chem. Commun. 2005, 3600–3602;
- 7cN. Kumagai, S. Matsunaga, M. Shibasaki, Tetrahedron 2007, 63, 8598–8608.
- 8L. Fan, O. V. Ozerov, Chem. Commun. 2005, 4450–4452.
- 9The pKa value for CH3CH(OH)CH3 is 30.3 in DMSO (W. N. Olmstead, Z. Margolin, F. G. Bordwell, J. Org. Chem. 1980, 45, 3295–3299). The pKa value of RCH(OH)CH2CN should be lower than 30.3 as a result of the electron-withdrawing nature of the CN group.
- 10
- 10aJ. M. Mayer, Comments Inorg. Chem. 1988, 8, 125–135;
- 10bK. G. Caulton, New J. Chem. 1994, 18, 25–41.
- 11Y. Suto, R. Tsuji, M. Kanai, M. Shibasaki, Org. Lett. 2005, 7, 3757–3760.
- 12
- 12aA. Goto, K. Endo, Y. Ukai, S. Irle, S. Saito, Chem. Commun. 2008, 2212–2214;
- 12bA. Goto, H. Naka, R. Noyori, S. Saito, Chem. Asian J. 2011, 6, 1740–1743.
- 13
- 13aS. Chakraborty, J. A. Krause, H. Guan, Organometallics 2009, 28, 582–586;
- 13bS. Chakraborty, J. Zhang, J. A. Krause, H. Guan, J. Am. Chem. Soc. 2010, 132, 8872–8873;
- 13cF. Huang, C. Zhang, J. Jiang, Z.-X. Wang, H. Guan, Inorg. Chem. 2011, 50, 3816–3825;
- 13dS. Chakraborty, Y. J. Patel, J. A. Krause, H. Guan, Polyhedron 2012, 32, 30–34;
- 13eS. Chakraborty, J. Zhang, Y. J. Patel, J. A. Krause, H. Guan, Inorg. Chem. 2013, 52, 37–47.
- 14
- 14aV. Pandarus, D. Zargarian, Chem. Commun. 2007, 978–980;
- 14bV. Pandarus, D. Zargarian, Organometallics 2007, 26, 4321–4334;
- 14cB. Vabre, M. L. Lambert, A. Petit, D. H. Ess, D. Zargarian, Organometallics 2012, 31, 6041–6053;
- 14dB. Vabre, D. M. Spasyuk, D. Zargarian, Organometallics 2012, 31, 8561–8570.
- 15This resonance becomes a broad singlet at δ=0.81 ppm when the sample is dissolved in CD2Cl2.
- 16
- 16aT. Naota, A. Tannna, S. Kamuro, M. Hieda, K. Ogata, S.-I. Murahashi, H. Takaya, Chem. Eur. J. 2008, 14, 2482–2498;
- 16bA. M. Oertel, V. Ritleng, M. J. Chetcuti, L. F. Veiros, J. Am. Chem. Soc. 2010, 132, 13588–13589;
- 16cA. M. Oertel, J. Freudenreich, J. Gein, V. Ritleng, L. F. Veiros, M. J. Chetcuti, Organometallics 2011, 30, 3400–3411;
- 16dT. A. Ateşin, T. Li, S. Lachaize, W. W. Brennessel, J. J. García, W. D. Jones, J. Am. Chem. Soc. 2007, 129, 7562–7569.
- 17CCDC 930777 (2) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18The adjustment of [PhCHO] was purely for the convenience of setting up the reaction without using too much CH3CN to dilute the catalyst concentration. For more details, see the Supporting Information.
- 19The pKa values (in H2O) for PhCH2OH and CH3CN are 15.4 and 25, respectively.
- 20
- 20aX. Lefèvre, G. Durieux, S. Lesturgez, D. Zargarian, J. Mol. Catal. A 2011, 335, 1–7;
- 20bX. Lefèvre, D. M. Spasyuk, D. Zargarian, J. Organomet. Chem. 2011, 696, 864–870.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.