Tightening of the Nanobelt upon Multielectron Reduction†
Dr. Alexander V. Zabula
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
New address: Department of Chemistry, University of Wisconsin, Madison, WI 53706 (USA)
Search for more papers by this authorDr. Alexander S. Filatov
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorDr. Jianlong Xia
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorCorresponding Author
Prof. Ramesh Jasti
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Ramesh Jasti, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Marina A. Petrukhina, Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marina A. Petrukhina
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Ramesh Jasti, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Marina A. Petrukhina, Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorDr. Alexander V. Zabula
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
New address: Department of Chemistry, University of Wisconsin, Madison, WI 53706 (USA)
Search for more papers by this authorDr. Alexander S. Filatov
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorDr. Jianlong Xia
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorCorresponding Author
Prof. Ramesh Jasti
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Ramesh Jasti, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Marina A. Petrukhina, Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marina A. Petrukhina
Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Ramesh Jasti, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Marina A. Petrukhina, Department of Chemistry, University at Albany, State University of New York, Albany, NY 12222 (USA)
Search for more papers by this authorFinancial support of this research by the National Science Foundation (CHE-1212441) is gratefully acknowledged by M.A.P. We thank the University at Albany for supporting the X-ray Center. R.J. also thanks Boston University for generous start-up funds.
Graphical Abstract
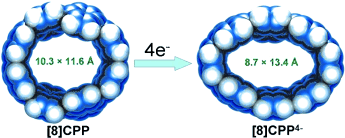
Den Gürtel enger schnallen: Die strukturelle Charakterisierung des mehrfach geladenen Fragments einer „Sessel“-Nanoröhre, das durch Reduktion des neutralen Moleküls mit Kalium gebildet wurde, offenbart eine bemerkenswerte elliptische Stauchung des Kohlenstoffrings (siehe Bild). Die entstandenen Tetraanionen bilden ein supramolekulares Aggregat mit Kaliumkationen, das solvatisierte Gastmoleküle aufnehmen kann.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201301226_sm_miscellaneous_information.pdf199.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. S. Filatov, L. T. Scott, M. A. Petrukhina, Cryst. Growth Des. 2010, 10, 4607–4621;
- 1b Fragments of Fullerenes and Nanotubes (Eds.: ), Wiley, Hoboken, 2012, p. 413;
- 1cD. Eisenberg, R. Shenhar, WIREs Comput. Mol. Sci. 2012, 2, 525–547.
- 2
- 2aL. T. Scott, H. E. Bronstein, D. V. Preda, R. B. M. Ansems, M. S. Bratcher, S. Hagen, Pure Appl. Chem. 1999, 71, 209–219;
- 2bV. M. Tsefrikas, L. T. Scott, Chem. Rev. 2006, 106, 4868–4884;
- 2cY.-T. Wu, J. S. Siegel, Chem. Rev. 2006, 106, 4843–4867;
- 2dA. Sygula, Eur. J. Org. Chem. 2011, 1611–1625.
- 3
- 3aA. Ayalon, M. Rabinovitz, P.-C. Cheng, L. T. Scott, Angew. Chem. 1992, 104, 1691–1692; Angew. Chem. Int. Ed. Engl. 1992, 31, 1636–1637;
- 3bM. Baumgarten, J. L. Gherghel, M. Wagner, A. Weitz, M. Rabinovitz, P.-C. Cheng, L. T. Scott, J. Am. Chem. Soc. 1995, 117, 6254–6257;
- 3cA. Weitz, E. Shabtai, M. Rabinovitz, M. S. Bratcher, C. C. McComas, M. D. Best, L. T. Scott, Chem. Eur. J. 1998, 4, 234–239;
- 3dI. Aprahamian, R. E. Hoffman, T. Sheradsky, D. V. Preda, M. Bancu, L. T. Scott, M. Rabinovitz, Angew. Chem. 2002, 114, 1788–1791;
10.1002/1521-3757(20020517)114:10<1788::AID-ANGE1788>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1712–1715;10.1002/1521-3773(20020517)41:10<1712::AID-ANIE1712>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 3eI. Aprahamian, D. V. Preda, M. Bancu, A. P. Belanger, T. Sheradsky, L. T. Scott, M. Rabinovitz, J. Org. Chem. 2006, 71, 290–298.
- 4
- 4aA. Ayalon, A. Sygula, P.-C. Cheng, M. Rabinovitz, P. W. Rabideau, L. T. Scott, Science 1994, 265, 1065–1067;
- 4bA. Weitz, M. Rabinovitz, P.-C. Cheng, L. T. Scott, Synth. Met. 1997, 86, 2159–2160;
- 4cE. Shabtai, R. E. Hoffman, P.-C. Cheng, E. Bayrd, D. V. Preda, L. T. Scott, M. Rabinovitz, J. Chem. Soc. Perkin Trans. 2 2000, 129–133;
- 4dN. Treitel, T. Sheradsky, L. Peng, L. T. Scott, M. Rabinovitz, Angew. Chem. 2006, 118, 3351–3355;
10.1002/ange.200503542 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3273–3277;
- 4eD. Eisenberg, J. M. Quimby, E. A. Jackson, L. T. Scott, R. Shenhar, Chem. Commun. 2010, 46, 9010–9012;
- 4fD. Eisenberg, E. A. Jackson, J. M. Quimby, L. T. Scott, R. Shenhar, Angew. Chem. 2010, 122, 7700–7704;
10.1002/ange.201002515 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7538–7542;
- 4gA. V. Zabula, A. S. Filatov, S. N. Spisak, A. Y. Rogachev, M. A. Petrukhina, Science 2011, 333, 1008–1011;
- 4hS. N. Spisak, A. V. Zabula, A. S. Filatov, A. Y. Rogachev, M. A. Petrukhina, Angew. Chem. 2011, 123, 8240–8244;
10.1002/ange.201103028 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8090–8094;
- 4iA. V. Zabula, S. N. Spisak, A. S. Filatov, M. A. Petrukhina, Angew. Chem. 2012, 124, 12360–12364;
10.1002/ange.201206936 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12194–12198;
- 4jA. V. Zabula, S. N. Spisak, A. S. Filatov, V. M. Grigoryants, M. A. Petrukhina, Chem. Eur. J. 2012, 18, 6476–6484;
- 4kA. V. Zabula, S. N. Spisak, A. S. Filatov, M. A. Petrukhina, Organometallics 2012, 31, 5541–5545;
- 4lD. Eisenberg, J. M. Quimby, L. T. Scott, R. Shenhar, J. Phys. Org. Chem. 2013, 26, 124–130;
- 4mS. N. Spisak, A. V. Zabula, M. V. Ferguson, A. S. Filatov, M. A. Petrukhina, Organometallics 2013, 32, 538–543.
- 5
- 5aG. Sandí, R. E. Gerald II, L. G. Scanlon, C. S. Johnson, R. J. Klingler, J. W. Rathke, J. New Mater. Electrochem. Syst. 2000, 3, 13–19;
- 5bR. E. Gerald II, R. J. Klingler, G. Sandi, G. Sandí, C. S. Johnson, L. G. Scanlon, J. W. Rathke, J. Power Sources 2000, 89, 237–243.
- 6
- 6aR. Jasti, C. R. Bertozzi, Chem. Phys. Lett. 2010, 494, 1–7;
- 6bH. Omachi, S. Matsuura, Y. Segawa, K. Itami, Angew. Chem. 2010, 122, 10400–10403; Angew. Chem. Int. Ed. 2010, 49, 10202–10205;
- 6cE. S. Hirst, R. Jasti, J. Org. Chem. 2012, 77, 10473–10478;
- 6dU. H. F. Bunz, S. Menning, N. Martín, Angew. Chem. 2012, 124, 7202–7209; Angew. Chem. Int. Ed. 2012, 51, 7094–7101.
- 7V. C. Parekh, P. C. Guha, J. Indian Chem. Soc. 1934, 11, 95–100.
- 8R. Friederich, M. Nieger, F. Vögtle, Chem. Ber. 1993, 126, 1723–1732.
- 9R. Jasti, J. Chattacharjee, J. B. Neaton, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 17646–17647.
- 10
- 10aY. Segawa, S. Miyamoto, H. Omachi, S. Matsuura, P. Senel, T. Sasamori, N. Tokitoh, K. Itami, Angew. Chem. 2011, 123, 3302–3306; Angew. Chem. Int. Ed. 2011, 50, 3244–3248;
- 10bJ. Xia, J. W. Bacon, R. Jasti, Chem. Sci. 2012, 3, 3018–3021.
- 11
- 11aH. Takaba, H. Omachi, Y. Yamamoto, J. Bouffard, K. Itami, Angew. Chem. 2009, 121, 6228–6232;
10.1002/ange.200902617 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6112–6116;
- 11bY. Ishii, Y. Nakanishi, H. Omachi, S. Matsuura, K. Matsui, H. Shinohara, Y. Segawa, K. Itami, Chem. Sci. 2012, 3, 2340–2345;
- 11cS. Yamago, Y. Watanabe, T. Iwamoto, Angew. Chem. 2010, 122, 769–771;
10.1002/ange.200905659 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 757–759;
- 11dT. Iwamoto, Y. Watanabe, Y. Sakamoto, T. Suzuki, S. Yamago, J. Am. Chem. Soc. 2011, 133, 8354–8361;
- 11eT. J. Sisto, M. R. Golder, E. S. Hirst, R. Jasti, J. Am. Chem. Soc. 2011, 133, 15800–15802;
- 11fJ. Xia, R. Jasti, Angew. Chem. 2012, 124, 2524–2526; Angew. Chem. Int. Ed. 2012, 51, 2474–2476;
- 11gE. R. Darzi, T. J. Sisto, R. Jasti, J. Org. Chem. 2012, 77, 6624–6628.
- 12
- 12aY. Segawa, A. Fukazawa, S. Matsuura, H. Omachi, S. Yamaguchi, S. Irle, K. Itami, Org. Biomol. Chem. 2012, 10, 5979–5984;
- 12bB. M. Wong, J. Phys. Chem. C 2009, 113, 21921–21927.
- 13T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino, S. Yamago, Angew. Chem. 2011, 123, 8492–8494; Angew. Chem. Int. Ed. 2011, 50, 8342–8344.
- 14We are only aware of one computational study: S. Taubert, D. Sundholm, F. Pichierri, J. Org. Chem. 2010, 75, 5867–5874.
- 15Synthesis of 14−/4 K+: THF (3 mL, dried over NaK2) was condensed into a glass tube containing [8]CPP (2.0 mg, 0.0035 mmol), potassium (5.0 mg, 0.128 mmol, excess), and [18]crown-6 (3.0 mg, 0.01 mmol). The tube was subsequently sealed under reduced pressure. A blue crystalline precipitate started forming on the metal surface at 25 °C within several days. Crystals of 14−/4 K+ (2.5 mg, 50 %) were separated manually from the metal surface after 3 weeks. UV/Vis (THF): λmax=600 nm. The very low solubility of 14−/4 K+ precluded the collection of satisfactory NMR spectroscopic data.
- 16X-ray crystal-structure determination of 14−/4 K+: C84H104K4O15, Mr=1510.07, 0.29×0.12×0.10 mm3, a=22.0146(19), b=23.057(2), c=15.4562(13) Å, β=94.5860(10)°, V=7820.3(12) Å3, ρcalcd=1.283 g cm−3, μ=0.292 mm−1, 33001 measured intensities (1.28°≤2θ≤50.0°, 99.6 % completeness), λ=0.71073 Å, T=100(2) K, 13719 independent reflections, monoclinic, P21/c, Z=4. The crystal was found to be a nonmerohedral twin. Subsequently, a TWINABS absorption correction was carried out (0.9200≤T≤0.9713), and the final refinement cycles were carried out against the HKLF 5 file with BASF 0.48824. R1=0.0944, wR2=0.1640 for [I≥2σ(I)] and R1=0.1283, wR2=0.1794 for all data. Goodness-of-fit on F2: 1.098, largest diff. peak and hole: 0.566 and −0.543 e Å3. All atoms were refined with anisotropic thermal parameters; hydrogen atoms were refined by using a riding model. CCDC 923514 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17
- 17aC. Näther, H. Bock, Z. Havlas, T. Hauck, Organometallics 1998, 17, 4707–4715;
- 17bC. Janiak, H. Hemling, Chem. Ber. 1994, 127, 1251–1253;
- 17cH. Bock, K. Gharagozloo-Hubmann, M. Sievert, T. Prisner, Z. Havlas, Nature 2000, 404, 267–269;
- 17dT. A. Scott, B. A. Ooro, D. J. Collins, M. Shatruk, A. Yakovenko, K. R. Dunbar, H.-C. Zhou, Chem. Commun. 2009, 65–67.
- 18
- 18aA. V. Zabula, S. N. Spisak, A. S. Filatov, A. Y. Rogachev, M. A. Petrukhina, Angew. Chem. 2011, 123, 3027–3030; Angew. Chem. Int. Ed. 2011, 50, 2971–2974;
- 18bA. V. Zabula, C. Dubceac, A. S. Filatov, M. A. Petrukhina, J. Org. Chem. 2011, 76, 9572–9576;
- 18cC. Dubceac, A. V. Zabula, A. S. Filatov, F. Rossi, P. Zanello, M. A. Petrukhina, J. Phys. Org. Chem. 2012, 25, 553–558.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.