A Cationic High-Valent Cp*CoIII Complex for the Catalytic Generation of Nucleophilic Organometallic Species: Directed CH Bond Activation†
Tatsuhiko Yoshino
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Search for more papers by this authorHideya Ikemoto
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Search for more papers by this authorCorresponding Author
Dr. Shigeki Matsunaga
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Japan Science and Technology Agency, ACT-C, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html===Search for more papers by this authorCorresponding Author
Prof. Dr. Motomu Kanai
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Japan Science and Technology Agency, ERATO, Kanai Life Science Catalysis Project, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html===Search for more papers by this authorTatsuhiko Yoshino
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Search for more papers by this authorHideya Ikemoto
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Search for more papers by this authorCorresponding Author
Dr. Shigeki Matsunaga
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Japan Science and Technology Agency, ACT-C, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html===Search for more papers by this authorCorresponding Author
Prof. Dr. Motomu Kanai
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html
Japan Science and Technology Agency, ERATO, Kanai Life Science Catalysis Project, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan) http://www.f.u-tokyo.ac.jp/∼kanai/e_index.html===Search for more papers by this authorThis research was supported in part by the ERATO program of JST (M.K.), a Grant-in-Aid for Scientific Research on Innovative Areas (“Molecular Activation Directed toward Straightforward Synthesis”) from MEXT (S.M.), the ACT-C program of JST (S.M.), and the Naito Foundation. T.Y. thanks the JSPS for fellowships. Cp*=pentamethylcyclopentadienyl.
Graphical Abstract
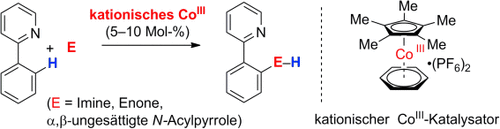
Aktiv ohne Aktivierung: In einem preiswerten und atomökonomischen Ansatz der C-H-Funktionalisierung wird ein kationischer Cobalt(III)-Komplex verwendet, um nucleophile metallorganische Spezies in situ ohne Zusatz aktivierender Reagentien herzustellen (siehe Schema). Aryl-C-H-Bindungen addieren effizient an polare Elektrophile, einschließlich α,β-ungesättigter N-Acylpyrrole als β-substituierte Ester- und Amid-Surrogate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209226_sm_miscellaneous_information.pdf5.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. M. Trost, Science 1991, 254, 1471.
- 2
- 2aV. Ritleng, C. Sirlin, M. Pfeffer, Chem. Rev. 2002, 102, 1731;
- 2bF. Kakiuchi, N. Chatani, Adv. Synth. Catal. 2003, 345, 1077;
- 2cK. Godula, D. Sames, Science 2006, 312, 67;
- 2dF. Kakiuchi, T. Kochi, Synthesis 2008, 3013;
- 2eL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. 2009, 121, 9976; Angew. Chem. Int. Ed. 2009, 48, 9792;
- 2fD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 2gT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 2hN. Yoshikai, Synlett 2011, 1047, and references therein.
- 3
- 3aY. Fukumoto, K. Sawada, M. Hagihara, N. Chatani, S. Murai, Angew. Chem. 2002, 114, 2903;
10.1002/1521-3757(20020802)114:15<2903::AID-ANGE2903>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2779;10.1002/1521-3773(20020802)41:15<2779::AID-ANIE2779>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 3bY. Kuninobu, Y. Nishina, C. Nakagawa, K. Takai, J. Am. Chem. Soc. 2006, 128, 12376;
- 3cY. Kuninobu, Y. Nishina, T. Takeuchi, K. Takai, Angew. Chem. 2007, 119, 6638;
10.1002/ange.200702256 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6518;
- 3dB.-J. Li, Z.-J. Shi, Chem. Sci. 2011, 2, 488;
- 3eK. Gao, N. Yoshikai, Chem. Commun. 2012, 48, 4305.
- 4Cp*RhIII complexes were originally utilized for oxidative CH bond functionalization; for reviews, see:
- 4aT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212;
- 4bG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651; for leading examples, see also:
- 4cK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407;
- 4dK. Ueura, T. Satoh, M. Miura, J. Org. Chem. 2007, 72, 5362; for oxidative reactions with polar multiple bonds, see also Refs. [6b, 8b,c]. Nonoxidative alkenylation with alkynes has also been reported:
- 4eD. Schipper, M. Hutchinson, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 6910.
- 5
- 5aA. S. Tsai, M. E. Tauchert, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2011, 133, 1248;
- 5bY. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen, Z.-J. Shi, Angew. Chem. 2011, 123, 2163; Angew. Chem. Int. Ed. 2011, 50, 2115;
- 5cM. E. Tauchert, C. D. Incarvito, A. L. Rheingold, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2012, 134, 1482;
- 5dY. Li, X.-S. Zhang, H. Li, W.-H. Wang, K. Chen, B.-J. Li, Z.-J. Shi, Chem. Sci. 2012, 3, 1634;
- 5eK. D. Hesp, R. G. Bergman, J. A. Ellman, Org. Lett. 2012, 14, 2304.
- 6
- 6aL. Yang, C. A. Correia, C.-J. Li, Adv. Synth. Catal. 2011, 353, 1269;
- 6bJ. Park, E. Park, A. Kim, Y. Lee, K.-W. Chi, J. H. Kwak, Y. H. Jung, I. S. Kim, Org. Lett. 2011, 13, 4390;
- 6cY. Li, X.-S. Zhang, K. Chen, K.-H. He, F. Pan, B.-J. Li, Z.-J. Shi, Org. Lett. 2012, 14, 636;
- 6dY. Lian, R. G. Bergman, J. A. Ellman, Chem. Sci. 2012, 3, 3088.
- 7
- 7aL. Yang, C. A. Correia, C.-J. Li, Org. Biomol. Chem. 2011, 9, 7176;
- 7bL. Yang, B. Qian, H. Huang, Chem. Eur. J. 2012, 18, 9511.
- 8
- 8aK. D. Hesp, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2011, 133, 11430;
- 8bC. Zhu, W. Xie, J. R. Falck, Chem. Eur. J. 2011, 17, 12591;
- 8cY. Du, T. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 12074.
- 9For the RuII-catalyzed addition of arenes to isocyanates, see: K. Muralirajan, K. Parthasarathy, C.-H. Cheng, Org. Lett. 2012, 14, 4262.
- 10Low-valent cobalt catalysts have been investigated intensively for CH functionalization reactions. For leading examples, see:
- 10aK. Gao, P.-S. Lee, T. Fujita, N. Yoshikai, J. Am. Chem. Soc. 2010, 132, 12249;
- 10bK. Gao, N. Yoshikai, J. Am. Chem. Soc. 2011, 133, 400;
- 10cQ. Chen, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 428;
- 10dB. Li, Z.-H. Wu, Y.-F. Gu, C.-L. Sun, B.-Q. Wang, Z.-J. Shi, Angew. Chem. 2011, 123, 1141; Angew. Chem. Int. Ed. 2011, 50, 1109;
- 10eZ. Ding, N. Yoshikai, Angew. Chem. 2012, 124, 4776; Angew. Chem. Int. Ed. 2012, 51, 4698;
- 10fW. Song, L. Ackermann, Angew. Chem. 2012, 124, 8376; Angew. Chem. Int. Ed. 2012, 51, 8251. For early examples, see Ref. [2h].
- 11For a review on CH bond activation/CC bond formation catalyzed by first-row transition metals, see: A. A. Kulkarni, O. Daugulis, Synthesis 2009, 4087.
- 12(2-Thiophenesulfonyl)imines were utilized in this study instead of p-toluenesulfonylimines because the 2-thiophenesulfonyl group in the products can be readily removed with Mg powder in MeOH (see Ref. [13]).
- 13For leading examples of the synthetic utility of (2-thiophenesulfonyl)imines and related heteroarenesulfonyl imines, see:
- 13aA. S. González, R. Gómez Arrayás, J. C. Carretero, Org. Lett. 2006, 8, 2977;
- 13bJ. Esquivias, R. Gómez Arrayás, J. C. Carretero, J. Am. Chem. Soc. 2007, 129, 1480;
- 13cH. Morimoto, G. Lu, N. Aoyama, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2007, 129, 9588;
- 13dS. Nakamura, H. Nakashima, H. Sugimoto, H. Sano, M. Hattori, N. Shibata, T. Toru, Chem. Eur. J. 2008, 14, 2145, and references therein.
- 14The p-toluenesulfonylimine derived from benzaldehyde was also a suitable substrate with the present Cp*CoIII complex 1 a. Its reaction with 2 a gave the desired product in 71 % yield.
- 15
- 15aE. O. Fischer, R. D. Fischer, Naturforsch. B 1961, 16, 556;
- 15bG. Fairhurst, C. White, J. Chem. Soc. Dalton Trans. 1979, 1531;
- 15cU. Kölle, B. Fuss, M. V. Rajasekharan, B. L. Ramakrishna, J. H. Ammeter, M. C. Böhm, J. Am. Chem. Soc. 1984, 106, 4152.
- 16For the preparation of the new cationic Co complexes 1 b–1 e, see the Supporting Information. We did not utilize the corresponding cationic CoIII catalyst with a simple Cp ligand because it was rather unstable.
- 17To enable the gram-scale synthesis of complex 1 a with a simple purification process, we modified the reported procedure in Ref. [15c]; see also: U. Kölle, B. Fuss, Chem. Ber. 1984, 117, 743.
- 18These reaction conditions were not applicable to imines derived from aliphatic aldehydes, possibly as a result of fast isomerization to the corresponding enamides.
- 19For the use of α,β-unsaturated N-acyl pyrroles as ester and amide surrogates, see:
- 19aT. Kinoshita, S. Okada, S.-R. Park, S. Matsunaga, M. Shibasaki, Angew. Chem. 2003, 115, 4828;
10.1002/ange.200352509 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4680;
- 19bS. Matsunaga, T. Kinoshita, S. Okada, M. Shibasaki, J. Am. Chem. Soc. 2004, 126, 7559;
- 19cR. Shintani, T. Kimura, T. Hayashi, Chem. Commun. 2005, 3213;
- 19dT. Mita, K. Sasaki, M. Kanai, M. Shinasaki, J. Am. Chem. Soc. 2005, 127, 514;
- 19eH. Komai, T. Yoshino, S. Matsunaga, M. Kanai, Org. Lett. 2011, 13, 1706.
- 20For a review on the use of other N-acyl pyrroles and related compounds in organic synthesis, see:
- 20aA. M. Goldys, C. S. P. McErlean, Eur. J. Org. Chem. 2012, 1877; for leading examples, see also:
- 20bD. A. Evans, G. Borg, K. A. Scheidt, Angew. Chem. 2002, 114, 3320;
Angew. Chem. Int. Ed. 2002, 41, 3188;
10.1002/1521-3773(20020902)41:17<3188::AID-ANIE3188>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 20cD. J. Dixon, M. S. Scott, C. A. Luckhurst, Synlett 2003, 2317;
- 20dS. Harada, S. Handa, S. Matsunaga, M. Shibasaki, Angew. Chem. 2005, 117, 4439;
10.1002/ange.200501180 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4365;
- 20eT. Maehara, R. Kanno, S. Yokoshima, T. Fukuyama, Org. Lett. 2012, 14, 1946;
- 20fB. M. Trost, W. M. Seganish, C. K. Chung, D. Amans, Chem. Eur. J. 2012, 18, 2948, and references therein.
- 21For the use of chiral Cp*Rh complexes for enantioselective transformations, see:
- 21aT. K. Hyster, L. Knörr, T. R. Ward, T. Rovis, Science 2012, 338, 500;
- 21bB. Ye, N. Cramer, Science 2012, 338, 504.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.