Halochromic Phenolate Perylene Bisimides with Unprecedented NIR Spectroscopic Properties
Dr. Mei-Jin Lin
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Search for more papers by this authorBenjamin Fimmel
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Search for more papers by this authorDr. Krzysztof Radacki
Universität Würzburg, Institut für Anorganische Chemie, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Würthner
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.deSearch for more papers by this authorDr. Mei-Jin Lin
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Search for more papers by this authorBenjamin Fimmel
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Search for more papers by this authorDr. Krzysztof Radacki
Universität Würzburg, Institut für Anorganische Chemie, Am Hubland, 97074 Würzburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Würthner
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.de
Universität Würzburg, Institut für Organische Chemie and Röntgen Research Center for Complex Material Systems, Am Hubland, 97074 Würzburg (Germany) http://www-organik.chemie.uni-wuerzburg.deSearch for more papers by this authorGraphical Abstract
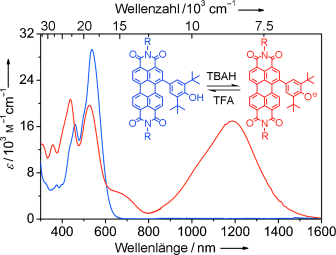
Die C-C-Kupplung von 1,7-Dibromperylenbisimid mit dem sterisch gehinderten 2,6-Di-tert-butylphenol durch eine nukleophile Substitution in Abwesenheit eines Übergangsmetall-Katalysators führte zu neuartigen halochromen Perylenbisimiden (siehe Bild). Die entsprechenden Phenolat-Ionen dieser Verbindungen zeigen neuartige NIR-Eigenschaften einschließlich starker Absorption mit Maxima um die 1200 nm.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105129_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. S. Schanze, J. R. Reynolds, J. M. Boncella, B. S. Harrison, T. J. Foley, M. Bouguettaya, T.-S. Kang, Synth. Met. 2003, 137, 1013–1014;
- 1bE. L. Williams, J. Li, G. E. Jabbour, Appl. Phys. Lett. 2006, 89, 083506.
- 2
- 2aE. E. Nesterov, J. Skoch, B. T. Hyman, W. E. Klunk, B. J. Bacskai, T. M. Swager, Angew. Chem. 2005, 117, 5588–5592;
10.1002/ange.200500845 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5452–5456;
- 2bM. Hintersteiner, A. Enz, P. Frey, A.-L. Jaton, W. Kinzy, R. Kneuer, U. Neumann, M. Rudin, M. Staufenbiel, M. Stoeckli, K. H. Wiederhold, H.-U. Gremlich, Nat. Biotechnol. 2005, 23, 577–583.
- 3
- 3aJ.-J. Cid, J. H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel, T. Torres, Angew. Chem. 2007, 119, 8510–8514;
10.1002/ange.200703106 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8358–8362;
- 3bH. Bürckstümmer, N. M. Kronenberg, K. Meerholz, F. Würthner, Org. Lett. 2010, 12, 3666–3669.
- 4
- 4aS. R. Marder, L.-T. Cheng, B. G. Tiemann, A. C. Friedli, M. Blanchard-Desce, J. W. Perry, J. Skindhøj, Science 1994, 263, 511–514;
- 4bZ. Y. Wang, J. Zhang, X. Wu, M. Birau, G. Yu, H. Yu, Y. Qi, P. Desjardins, X. Meng, J. P. Gao, E. Todd, N. Song, Y. Bai, A. M. R. Beaudin, G. LeClair, Pure Appl. Chem. 2004, 76, 1435–1443.
- 5For selected NIR-absorbing cyanines, see:
- 5aJ. Fabian, J. Prakt. Chem. 1991, 333, 197–222;
- 5bS. Ohira, J. M. Hales, K. J. Thorley, H. L. Anderson, J. W. Perry, J.-L. Brédas, J. Am. Chem. Soc. 2009, 131, 6099–6101.
- 6For selected NIR-absorbing squaraines, see:
- 6aA. Ajayaghosh, Acc. Chem. Res. 2005, 38, 449–459;
- 6bU. Mayerhöffer, K. Deing, K. Gruß, H. Braunschweig, K. Meerholz, F. Würthner, Angew. Chem. 2009, 121, 8934–8937;
10.1002/ange.200903125 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8776–8779.
- 7For selected NIR-absorbing Bodipys, see:
- 7aA. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891–4932;
- 7bG. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. 2008, 120, 1202–1219;
10.1002/ange.200702070 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1184–1201;
- 7cR. Gresser, M. Hummert, H. Hartmann, K. Leo, M. Riede, Chem. Eur. J. 2011, 17, 2939–2947.
- 8For selected NIR-absorbing porphyrins, see:
- 8aA. Jasat, D. Dolphin, Chem. Rev. 1997, 97, 2267–2340;
- 8bA. Tsuda, A. Osuka, Science 2001, 293, 79–82.
- 9For selected NIR-absorbing phthalocyanines, see:
- 9aC. G. Claessens, U. Hahn, T. Torres, Chem. Rec. 2008, 8, 75–97;
- 9bJ. Mack, N. Kobayashi, Chem. Rev. 2011, 111, 281–321.
- 10For selected NIR-absorbing rylene dyes, see:
- 10aN. G. Pschirer, C. Kohl, F. Nolde, J. Qu, K. Müllen, Angew. Chem. 2006, 118, 1429–1432;
10.1002/ange.200502998 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1401–1404;
- 10bH. Qian, Z. Wang, W. Yue, D. Zhu, J. Am. Chem. Soc. 2007, 129, 10664–10665;
- 10cY. Li, J. Gao, S. Di Motta, F. Negri, Z. Wang, J. Am. Chem. Soc. 2010, 132, 4208–4213.
- 11
- 11aJ. Fabian, H. Nakazumi, M. Matsuoka, Chem. Rev. 1992, 92, 1197–1226;
- 11bG. Qian, Z. Y. Wang, Chem. Asian J. 2010, 5, 1006–1029;
- 11cT. Weil, T. Vosch, J. Hofkens, K. Peneva, K. Müllen, Angew. Chem. 2010, 122, 9252–9278; Angew. Chem. Int. Ed. 2010, 49, 9068–9093.
- 12
- 12aH. Langhals, Heterocycles 1995, 40, 477–500;
- 12bF. Würthner, Chem. Commun. 2004, 1564–1579;
- 12cC. Huang, S. Barlow, S. R. Marder, J. Org. Chem. 2011, 76, 2386–2407.
- 13
- 13aC.-C. Chao, M.-K. Leung, Y. O. Su, K.-Y. Chiu, T.-H. Lin, S.-J. Shieh, S.-C. Lin, J. Org. Chem. 2005, 70, 4323–4331;
- 13bJ. Huang, H. Fu, Y. Wu, S. Chen, F. Shen, X. Zhao, Y. Liu, J. Yao, J. Phys. Chem. C 2008, 112, 2689–2696;
- 13cJ. Huang, Y. Wu, H. Fu, X. Zhan, J. Yao, S. Barlow, S. R. Marder, J. Phys. Chem. A 2009, 113, 5039–5046.
- 14
- 14aS. Shoaee, Z. An, X. Zhang, S. Barlow, S. R. Marder, W. Duffy, M. Heeney, I. McCulloch, J. R. Durrant, Chem. Commun. 2009, 5445–5447;
- 14bZ. An, S. A. Odom, R. F. Kelley, C. Huang, X. Zhang, S. Barlow, L. A. Padilha, J. Fu, S. Webster, D. J. Hagan, E. W. Van Stryland, M. R. Wasielewski, S. R. Marder, J. Phys. Chem. A 2009, 113, 5585–5593.
- 15
- 15aN. Isaacs, Physical Organic Chemistry, 2nd ed., Longman Scientific, Essex, 1995;
- 15bI. G. Binev, R. B. Kuzmanova, J. Kaneti, I. N. Juchnovski, J. Chem. Soc. Perkin Trans. 2 1982, 1533–1536.
- 16
- 16aA. Böhm, H. Arms, G. Henning, P. Blaschka, German Pat. DE 19547209A1, 1997 [Chem. Abstr. 1997, 127, 96569g];
- 16bF. Würthner, V. Stepanenko, Z. Chen, C. R. Saha-Möller, N. Kocher, D. Stalke, J. Org. Chem. 2004, 69, 7933–7939.
- 17
- 17aG. P. Stahly, J. Org. Chem. 1985, 50, 3091–3094;
- 17bM. Brewis, G. J. Clarkson, P. Humberstone, S. Makhseed, N. B. McKeown, Chem. Eur. J. 1998, 4, 1633–1640.
10.1002/(SICI)1521-3765(19980904)4:9<1633::AID-CHEM1633>3.0.CO;2-O CAS Web of Science® Google Scholar
- 18
- 18aN. Alam, C. Amatore, C. Combellas, A. Thiébault, J. N. Verpeaux, Tetrahedron Lett. 1987, 28, 6171–6174;
- 18bR. Beugelmans, M. Bois-houssy, Tetrahedron Lett. 1988, 29, 1289–1292.
- 19
- 19aJ. Salbeck, H. Kunkely, H. Langhals, R. W. Saalfrank, J. Daub, Chimia 1989, 43, 6–9;
- 19bF. Würthner, A. Sautter, D. Schmid, P. J. A. Weber, Chem. Eur. J. 2001, 7, 894–902.
10.1002/1521-3765(20010216)7:4<894::AID-CHEM894>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 20
- 20aJ. F. Bunnett, R. G. Scamehorn, R. P. Traber, J. Org. Chem. 1976, 41, 3677–3682;
- 20bJ. F. Bunnett, Acc. Chem. Res. 1978, 11, 413–420.
- 21P. Osswald, F. Würthner, J. Am. Chem. Soc. 2007, 129, 14319–14326.
- 22M. M. Safont-Sempere, P. Osswald, M. Stolte, M. Grüne, M. Renz, M. Kaupp, K. Radacki, H. Braunschweig, F. Würthner, J. Am. Chem. Soc. 2011, 133, 9580–9591.
- 23
- 23aC. Reichardt, Chem. Rev. 1994, 94, 2319–2358;
- 23bC. Reichardt, Solvents and Solvent Effects in Organic Chemistry, 3rd ed., Wiley-VCH, Weinheim, 2003. For a profound discussion on halochromic phenomena, see: C. Reichardt, E. Harbusch-Görnert, G. Schäfer, Liebigs Ann. Chem. 1988, 839–844.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.