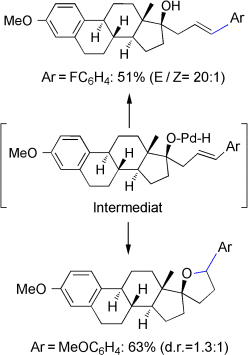
Alternative Pathways for Heck Intermediates: Palladium-Catalyzed Oxyarylation of Homoallylic Alcohols†
Corresponding Author
Dr. Chen Zhu
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455Search for more papers by this authorProf. Dr. John R. Falck
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455
Search for more papers by this authorCorresponding Author
Dr. Chen Zhu
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455Search for more papers by this authorProf. Dr. John R. Falck
Departments of Biochemistry and Pharmacology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9038 (USA), Fax: (+1) 214-648-6455
Search for more papers by this authorWe thank the NIH (GM31278) and the Robert A. Welch Foundation (GL625910) for financial support.
Graphical Abstract
Die Koordination der Hydroxygruppe an den Katalysator ist einer der Hauptgründe für die guten Ausbeuten und Regioselektivitäten in der oxidativen Heck-Arylierung von Homoallylalkoholen (siehe Schema). Ferner lässt sich das Heck-Intermediat durch eine intramolekular palladiumkatalysierte Olefin-Oxycyclisierung abfangen, die den Weg zu α-Aryltetrahydrofuranen mit hoch funktionalisierten Molekülgerüsten ebnet.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201101857_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aC. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 2001, 34, 633;
- 1bE. M. Beccalli, G. Broggini, M. Martinelli, S. Sottocornola, Chem. Rev. 2007, 107, 5318;
- 1cX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 1dT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147.
- 2For reviews, see:
- 2aR. F. Heck, Comprehensive Organic Synthesis, Vol. 4 (Ed.: ), Pergamon, New York, 1991, chap. 4.3;
10.1016/B978-0-08-052349-1.00110-4 Google Scholar
- 2bW. Cabri, I. Candiani, Acc. Chem. Res. 1995, 28, 2;
- 2cG. T. Crisp, Chem. Soc. Rev. 1998, 27, 427;
- 2dN. J. Whitcombe, K. K. Hii, S. E. Gibson, Tetrahedron 2001, 57, 7449;
- 2eA. B. Dounay, L. E. Overman, Chem. Rev. 2003, 103, 2945;
- 2fF. Alonso, I. P. Beletskaya, M. Yus, Tetrahedron 2005, 61, 11771;
- 2gB. Karimi, H. Behzadnia, D. Elhamifar, P. F. Akhavan, F. K. Esfahani, A. Zamani, Synthesis 2010, 1399.
- 3Other examples involving the trapping of a σ-alkyl palladium(II) Heck intermediate, see:
- 3aK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. 2006, 118, 7292;
10.1002/ange.200601872 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7134, and references therein;
- 3bY. Tamaru, M. Hojo, H. Higashimura, Z. Yoshida, Angew. Chem. 1986, 98, 740; Angew. Chem. Int. Ed. Engl. 1986, 25, 735;
- 3cY. Tamaru, M. Hojo, S. Kawamura, Z. Yoshida, J. Org. Chem. 1986, 51, 4089;
- 3dD. Kalyani, M. S. Sanford, J. Am. Chem. Soc. 2008, 130, 2150;
- 3eK. B. Urkalan, M. S. Sigman, Angew. Chem. 2009, 121, 3192;
10.1002/ange.200900218 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3146;
- 3fE. W. Werner, K. B. Urkalan, M. S. Sigman, Org. Lett. 2010, 12, 2848;
- 3gD. Kalyani, A. D. Satterfield, M. S. Sanford, J. Am. Chem. Soc. 2010, 132, 8419;
- 3hA. D. Satterfield, A. Kubota, M. S. Sanford, Org. Lett. 2011, 13, 1076.
- 4For the functionalization of olefins by CH bond activation, see: C. Zhu, J. R. Falck, Org. Lett. 2011, 13, 1214.
- 5For another strategy to construct tetrahydrofuran by palladium(0)-mediated olefin insertion into a PdO bond, see:
- 5aJ. P. Wolfe, Synlett 2008, 2913;
- 5bJ. P. Wolfe, M. A. Rossi, J. Am. Chem. Soc. 2004, 126, 1620;
- 5cM. B. Hay, J. P. Wolfe, J. Am. Chem. Soc. 2005, 127, 16468;
- 5dM. B. Hay, A. R. Hardin, J. P. Wolfe, J. Org. Chem. 2005, 70, 3099;
- 5eJ. S. Nakhla, J. W. Kampf, J. P. Wolfe, J. Am. Chem. Soc. 2006, 128, 2893;
- 5fA. F. Ward, J. P. Wolfe, Org. Lett. 2009, 11, 2209;
- 5gA. F. Ward, J. P. Wolfe, Org. Lett. 2010, 12, 1268.
- 6For examples of oxidative Heck reactions involving chelating groups, see:
- 6aJ. H. Delcamp, M. C. White, J. Am. Chem. Soc. 2006, 128, 15076;
- 6bJ. Ruan, X. Li, O. Saidi, J. Xiao, J. Am. Chem. Soc. 2008, 130, 2424;
- 6cD. Pan, A. Chen, Y. Su, W. Zhou, S. Li, W. Jia, J. Xiao, Q. Liu, L. Zhang, N. Jiao, Angew. Chem. 2008, 120, 4807; Angew. Chem. Int. Ed. 2008, 47, 4729;
- 6dJ. H. Delcamp, A. P. Brucks, M. C. White, J. Am. Chem. Soc. 2008, 130, 11270;
- 6eY. Su, N. Jiao, Org. Lett. 2009, 11, 2980.
- 7Excess TFA dehydrated the starting material resulting in diminished yields.
- 8For details, see the Supporting Information.
- 9The relative configuration was determined by 1H NMR, see:
- 9aG. Dana, E. Touboul, O. Convert, Y. L. Pascal, Tetrahedron 1988, 44, 429;
- 9bA. Schmitt, H. Reissig, Eur. J. Org. Chem. 2000, 3893.
- 10Because of the higher dissociation energy of a CD bond than a CH bond, the β-hydride elimination becomes the competitive reaction to the β-deuteride elimination, thus resulting in the retention of 36 % deuterium at α-carbon atom.
- 11For examples, see:
- 11aM. M. Konnick, B. A. Gandhi, I. A. Guzei, S. S. Stahl, Angew. Chem. 2006, 118, 2970;
10.1002/ange.200600532 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2904;
- 11bM. C. Denney, N. A. Smythe, K. L. Cetto, R. A. Kemp, K. I. Goldberg, J. Am. Chem. Soc. 2006, 128, 2508;
- 11cB. V. Popp, S. S. Stahl, J. Am. Chem. Soc. 2007, 129, 4410;
- 11dM. M. Konnick, S. S. Stahl, J. Am. Chem. Soc. 2008, 130, 5753.
- 12For examples, see:
- 12aM. S. Chen, N. Prabagaran, N. A. Labenz, M. C. White, J. Am. Chem. Soc. 2005, 127, 6970;
- 12bA. N. Campbell, P. B. White, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2010, 132, 15116.
- 13J. A. Mueller, C. P. Goller, M. S. Sigman, J. Am. Chem. Soc. 2004, 126, 9724.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.