Synthesis of ent-Nanolobatolide†
Dr. Hau Man Cheng
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Weiwei Tian
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Philippe A. Peixoto
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Bhartesh Dhudshia
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorCorresponding Author
Prof. Dr. David Y.-K. Chen
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869Search for more papers by this authorDr. Hau Man Cheng
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Weiwei Tian
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Philippe A. Peixoto
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorDr. Bhartesh Dhudshia
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Search for more papers by this authorCorresponding Author
Prof. Dr. David Y.-K. Chen
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869
Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, The Helios Block, #03-08, Singapore 138667 (Singapore), Fax: (+65) 6874-5869Search for more papers by this authorWe thank Doris Tan (ICES) for assistance with high-resolution mass spectrometry (HRMS). Financial support for this work was provided by A*STAR, Singapore.
Graphical Abstract
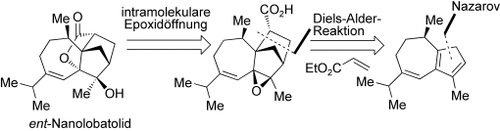
Effizient und anpassbar: Die Schlüsselschritte bei der Totalsynthese von ent-Nanolobatolid, dem Enantiomer des neuartigen und wirksamen neuroprotektiven Stoffes, sind eine oxidative Ringerweiterung von (−)-Menthon, eine Nazarov-Cyclisierung, eine intermolekulare Diels-Alder-Reaktion und eine intramolekulare Epoxidöffnung (siehe Schema). Die beiden letzten Schritte stützen den vermuteten Biosyntheseweg.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100926_sm_miscellaneous_information.pdf728 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. Esposito, S. Cuzzocrea, Curr. Med. Chem. 2010, 17, 2764–2774;
- 1bM. M. Husain, K. Trevino, H. Siddique, S. M. McClintock, Neuropsychiatr. Dis. Treat. 2008, 4, 765–777;
- 1cM. R. Loizzo, R. Tundis, F. Menichini, F. Menichini, Curr. Med. Chem. 2008, 15, 1209–1228; A. Lleo, Curr. Genomics 2007, 8, 550–558;
- 1dP. I. Moreira, X. Zhu, A. Nunomura, M. A. Smith, G. Perry, Expert Rev. Neurother. 2006, 6, 897–910;
- 1eC. H. Dowding, C. L. Shenton, S. S. Salek, Drugs Aging 2006, 23, 693–721;
- 1fR. M. Wilson, S. J. Danishefsky, Acc. Chem. Res. 2006, 39, 539–549.
- 2Y.-J. Tseng, Z.-H. Wen, C.-F. Dai, M. Y. Chiang, J.-H. Sheu, Org. Lett. 2009, 11, 5030–5032.
- 3
- 3aK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. 2002, 114, 1742–1773;
10.1002/1521-3757(20020517)114:10<1742::AID-ANGE1742>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1668–1698; for the Diels–Alder reaction of cyclopentadiene containing guaiane sesquiterpenes, see:10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 3bG. Lian, B. Yu, Chem. Biodiversity 2010, 7, 2660–2691;
- 3cT. A. Geissman, T. E. Winters, Tetrahedron Lett. 1968, 9, 3145–3147;
10.1016/S0040-4039(00)89574-X Google Scholar
- 3dK. Vokác, Z. Samek, V. Herout, F. Sorm, Tetrahedron Lett. 1968, 9, 3855–3857;
10.1016/S0040-4039(01)99119-1 Google Scholar
- 3eJ. Beauhaire, J. L. Fourrey, M. Vuilhorgne, J. Y. Lallemand, Tetrahedron Lett. 1980, 21, 3191–3194.
- 4
- 4aC. Santelli-Rouvier, M. Santelli, Synthesis 1983, 429–442;
- 4bS. E. Denmark, Comp. Org. Synth., Vol. 5, Pergamon Press, Oxford, 1991, pp. 751–784;
10.1016/B978-0-08-052349-1.00138-4 Google Scholar
- 4cK. L. Habermas, S. E. Denmark, T. K. Jones, Org. React. 1994, 45, 1–158.
- 5
- 5aH. E. Simmons, T. L. Cairns, S. A. Vladuchick, C. M. Holness, Org. React. 1973, 20, 1–131;
- 5bM. J. Conia, Pure Appl. Chem. 1975, 43, 317–326;
- 5cA. B. Charette, Organozinc Reagents 1999, 263–285;
- 5dA. B. Charette, A. Beauchemin, Org. React. 2001, 58, 1–415;
- 5eW. A. Donaldson, Tetrahedron 2001, 57, 8589–8627.
- 6aJ. Jiao, L. X. Nguyen, D. R. Patterson, R. A. Flowers II, Org. Lett. 2007, 9, 1323–1326;
- 6bK. Kakiuchi, M. Ue, H. Tsukahara, T. Shimizu, T. Miyao, Y. Tobe, Y. Odaira, M. Yasuda, K. Shima, J. Am. Chem. Soc. 1989, 111, 3707–3712.
- 7
- 7aN. Hayashi, M. Nakada, Tetrahedron Lett. 2009, 50, 232–235;
- 7bJ. R. Scheerer, J. F. Lawrence, G. C. Wang, D. A. Evans, J. Am. Chem. Soc. 2007, 129, 8968–8969.
- 8Attempts to install the C5–C6 olefin from ketone 16 through the corresponding enol triflate, enol phosphate, vinyl halide, and hydrazone all proved unsuccessful, leading to either recovery or extensive decomposition of the starting material 16.
- 9Attempts to eliminate alcohol 17 through its corresponding triflate, selenide, halide, xanthate, or under Mitsunobu, Martin’s sulfurane, Burgess reagent, and POCl3/SOCl2–pyridine conditions all proved ineffective for this process.
- 10Notably, although (+)-menthone, which would lead to the naturally occurring form of (+)-nanolobatolide (1) in accordance to our synthetic strategy, is less readily available and more expensive than (−)-menthone, both enantiomeric forms of menthol are readily available, and this compound can be easily oxidized to give (+) and (−)-menthone.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.