Gold-Catalyzed Hydroamination of Alkynes and Allenes with Parent Hydrazine†
Dr. Rei Kinjo
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorCorresponding Author
Prof. Guy Bertrand
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/Search for more papers by this authorDr. Rei Kinjo
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorCorresponding Author
Prof. Guy Bertrand
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
UCR-CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California Riverside, Riverside, CA 92521-0403 (USA), Fax: (+1) 202-354-5267 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/Search for more papers by this authorWe are grateful to the DOE (DE-FG02-09ER16069) for financial support of this research, and to the Japan Society for the Promotion of Science for fellowships (R.K.).
Graphical Abstract
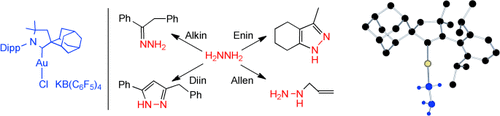
Bei der Addition von Hydrazin an Alkine, Diine, Enine und Allene in Gegenwart eines kationischen Gold(I)-Komplexes mit einem cyclischen (Alkyl)(amino)carben-Liganden bilden sich unterschiedliche stickstoffhaltige Verbindungen (siehe Schema; gezeigt ist auch die Molekülstruktur des Gold-Hydrazin-Komplexes im Kristall). Diese Hydroaminierung ist ein idealer erster Schritt für die Synthese acyclischer und heterocyclischer Grundchemikalien. Dipp=2,6-Diisopropylphenyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100740_sm_miscellaneous_information.pdf204 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. O’Hagan, Nat. Prod. Rep. 2000, 17, 435–446;
- 1bA. Mitchenson, A. Nadin, J. Chem. Soc. Perkin Trans. 1 2000, 2862–2892;
- 1cJ. W. Daly, H. M. Garraffo, T. F. Spande in The Alkaloids: Chemistry and Pharmacology, Vol. 43 (Ed.: ), Academic Press, San Diego, 1993, pp. 185–288;
- 1dA. Glisan King, J. Meinwald, Chem. Rev. 1996, 96, 1105–1122;
- 1eJ. W. Daly, Proc. Natl. Acad. Sci. USA 1995, 92, 9;
- 1fA. Brossi, The Alkaloids: Chemistry and Pharmacology, Vol. 43 (Ed.: ), Academic Press, San Diego, 1993, pp. 119–183.
- 2S. A. Lawrence, Amines: Synthesis, Properties, and Applications, Cambridge University Press, New York, 2004.
- 3For reviews on hydroamination reactions, see:
- 3aK. D. Hesp, M. Stradiotto, ChemCatChem 2010, 2, 1192–1207;
- 3bY. Fukumoto, J. Synth. Org. Chem. Jpn. 2009, 67, 735–750;
- 3cT. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795–3892;
- 3dR. Severin, S. Doye, Chem. Soc. Rev. 2007, 36, 1407–1420;
- 3eI. Aillaud, J. Collin, J. Hannedouche, E. Schulz, Dalton Trans. 2007, 5105–5118;
- 3fS. Matsunaga, J. Synth. Org. Chem. Jpn. 2006, 64, 778–779;
- 3gP. W. Roesky, Z. Anorg. Allg. Chem. 2006, 632, 1918–1926;
- 3hA. L. Odom, Dalton Trans. 2005, 225–233;
- 3iN. Hazari, P. Mountford, Acc. Chem. Res. 2005, 38, 839–849;
- 3jJ. F. Hartwig, Pure Appl. Chem. 2004, 76, 507–516;
- 3kS. Hong, T. J. Marks, Acc. Chem. Res. 2004, 37, 673–686;
- 3lM. Beller, J. Seayad, A. Tillack, H. Jiao, Angew. Chem. 2004, 116, 3448–3479;
10.1002/ange.200300616 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3368–3398;
- 3mM. Beller, A. Tillack, J. Seayad, Transition Metals for Organic Synthesis, 2nd ed., Wiley-VCH, Weinheim, 2004, pp. 403–414.
10.1002/9783527619405.ch5j Google Scholar
- 4R. J. Lundgren, M. Stradiotto, Angew. Chem. 2010, 122, 8868–8872;
10.1002/ange.201003764 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8686–8690.
- 5
- 5aB. T. Heaton, C. Jacob, P. Page, Coord. Chem. Rev. 1996, 154, 193–229;
- 5bJ. L. Klinkenberg, J. F. Hartwig, Angew. Chem. 2011, 123, 88–98; Angew. Chem. Int. Ed. 2011, 50, 86–95;
- 5cJ. I. van der Vlugt, Chem. Soc. Rev. 2010, 39, 2302–2322.
- 6
- 6aN. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Elsevier, Oxford, 1998, pp. 427–431;
- 6b“Hydrazine”: B. A. Roden in Encyclopedia of Reagents for Organic Synthesis (Ed.: ), Wiley, Chester, 2001;
- 6cP. P. Cellier, J. F. Spindler, M. Taillefer, H. J. Cristau, Tetrahedron Lett. 2003, 44, 7191–7195;
- 6dA. Furst, R. C. Berlo, S. Hooton, Chem. Rev. 1965, 65, 51–68.
- 7
- 7aJ. P. Chen, L. L. Lim, Chemosphere 2002, 49, 363–370;
- 7bD. K. Simpson, Metal Finishing 1985, 83, 57–60.
- 8
- 8aA. Dhakshinamoorthy, M. Alvaro, H. Garcia, Adv. Synth. Catal. 2009, 351, 2271–2276;
- 8bC. Smit, M. W. Fraaije, A. J. Minnaard, J. Org. Chem. 2008, 73, 9482–9485.
- 9For examples, see:
- 9aK.-S. Lee, Y.-K. Lim, C.-G. Cho, Tetrahedron Lett. 2002, 43, 7463–7464;
- 9bG. J. Ellames, J. S. Gibson, J. M. Herbert, A. H. McNeill, Tetrahedron 2001, 57, 9487–9497;
- 9cF. Alonso, G. Radivoy, M. Yus, Tetrahedron 2000, 56, 8673–8678.
- 10
- 10aR. L. LaLonde, Z. J. Wang, M. Mba, A. D. Lackner, F. D. Toste, Angew. Chem. 2010, 122, 608–611;
10.1002/ange.200905000 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 598–601;
- 10bZ. J. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard III, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 13064–13071;
- 10cS. Kramer, J. L. H. Madsen, M. Rottländer, T. Skrydsrup, Org. Lett. 2010, 12, 2758–2761;
- 10dS. L. Dabb, B. A. Messerle, Dalton Trans. 2008, 6368–6371;
- 10eA. M. Johns, Z. Liu, J. F. Hartwig, Angew. Chem. 2007, 119, 7397–7399;
10.1002/ange.200701899 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7259–7261;
- 10fI. A. Sayyed, K. Alex, A. Tillack, N. Schwarz, D. Michalik, M. Beller, Eur. J. Org. Chem. 2007, 4525–4528;
- 10gN. T. Patil, L. M. Lutete, N. Nishina, Y. Yamamoto, Tetrahedron Lett. 2006, 47, 4749–4751;
- 10hY. Li, Y. Shi, A. L. Odom, J. Am. Chem. Soc. 2004, 126, 1794–1803;
- 10iL. Ackermann, R. Born, Tetrahedron Lett. 2004, 45, 9541–9544;
- 10jE. Mizushima, T. Hayashi, M. Tanaka, Org. Lett. 2003, 5, 3349–3352;
- 10kC. Cao, Y. Shi, A. L. Odom, Org. Lett. 2002, 4, 2853–2856;
- 10lY. Fukumoto, T. Dohi, H. Masaoka, N. Chatani, S. Murai, Organometallics 2002, 21, 3845–3847;
- 10mN. Halland, M. Nazaré, J. Alonso, O. R’kyek, A. Lindenschmidt, Chem. Commun. 2010, 46, 1042–1044;
- 10nK. Weitershaus, H. Wadepohl, L. H. Gade, Organometallics 2009, 28, 3381–3389.
- 11
- 11aV. Lavallo, G. D. Frey, S. Kousar, B. Donnadieu, G. Bertrand, Proc. Natl. Acad. Sci. USA 2007, 104, 13569–13573;
- 11bG. D. Frey, R. D. Dewhurst, S. Kousar, B. Donnadieu, G. Bertrand, J. Organomet. Chem. 2008, 693, 1674–1682.
- 12
- 12aV. Lavallo, Y. Canac, C. Präsang, B. Donnadieu, G. Bertrand, Angew. Chem. 2005, 117, 5851–5855;
10.1002/ange.200501841 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5705–5709;
- 12bV. Lavallo, Y. Canac, A. DeHope, B. Donnadieu, G. Bertrand, Angew. Chem. 2005, 117, 7402–7405;
10.1002/ange.200502566 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7236–7239;
- 12cR. Jazzar, R. D. Dewhurst, J.-B. Bourg, B. Donnadieu, Y. Canac, G. Bertrand, Angew. Chem. 2007, 119, 2957–2960;
10.1002/ange.200605083 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2899–2902;
- 12dR. Jazzar, J.-B. Bourg, R. D. Dewhurst, B. Donnadieu, G. Bertrand, J. Org. Chem. 2007, 72, 3492–3499.
- 13
- 13aX. Zeng, R. Kinjo, B. Donnadieu, G. Bertrand, Angew. Chem. 2010, 122, 954–957; Angew. Chem. Int. Ed. 2010, 49, 942–945;
- 13bX. Zeng, G. D. Frey, R. Kinjo, B. Donnadieu, G. Bertrand, J. Am. Chem. Soc. 2009, 131, 8690–8696;
- 13cX. Zeng, G. D. Frey, S. Kousar, G. Bertrand, Chem. Eur. J. 2009, 15, 3056–3060;
- 13dX. Zeng, M. Soleilhavoup, G. Bertrand, Org. Lett. 2009, 11, 3166–3169;
- 13eV. Lavallo, G. D. Frey, B. Donnadieu, M. Soleilhavoup, G. Bertrand, Angew. Chem. 2008, 120, 5302–5306;
10.1002/ange.200801136 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5224–5228.
- 14For recent reviews on gold-catalyzed hydroamination, see:
- 14aA. S. K. Hashmi, Angew. Chem. 2010, 122, 5360–5369;
10.1002/ange.200907078 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5232–5241;
- 14bS. Sengupta, X. D. Shi, ChemCatChem 2010, 2, 609–619;
- 14cZ. Li, C. Brouwer, C. He, Chem. Rev. 2008, 108, 3239–3265;
- 14dA. Arcadi, Chem. Rev. 2008, 108, 3266–3325;
- 14eD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 14fN. T. Patil, Y. Yamamoto, Chem. Rev. 2008, 108, 3395–3442;
- 14gR. A. Widenhoefer, Chem. Eur. J. 2008, 14, 5382–5391;
- 14hN. Krause, V. Belting, C. Deutsch, J. Erdsack, H. T. Fan, B. Gockel, A. Hoffmann-Roder, N. Morita, F. Volz, Pure Appl. Chem. 2008, 80, 1063–1069;
- 14iA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478–3519;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410–3449;
- 14jR. A. Widenhoefer, X. Han, Eur. J. Org. Chem. 2006, 4555–4563.
- 15For examples, see:
- 15aX.-F. Yang, A.-Q. Wang, Y.-L. Wang, T. Zhang, J. Li, J. Phys. Chem. C 2010, 114, 3131–3139;
- 15bA. Pal, S. Shah, S. Devi, Colloids Surf. A 2007, 302, 51–57.
- 16H. Shan, Y. Yang, A. J. James, P. R. Sharp, Science 1997, 275, 1460–1462.
- 17CCDC 803848 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18For a thermally induced Cope-type hydroamination of alkynes with methyl hydrazine, see: P. H. Cebrowski, J.-G. Roveda, J. Moran, S. I. Gorelsky, A. M. Beauchemin, Chem. Commun. 2008, 492–493.
- 19One of the referees suggested that an alkyne-hydration/hydrazone-formation pathway could also be operating under the reaction conditions. However, as observed with other gold catalysts,[21] the addition of a trace amount of water to 1 g in the presence of A (5 mol %) cleanly led to 2,5-diphenylfuran and thus ruled out this hypothesis.
- 20A. Nakhai, J. Bergman, Tetrahedron 2009, 65, 2298–2306.
- 21aN. T. Patil, A. Konala, Eur. J. Org. Chem. 2010, 6831–6839;
- 21bP. Nun, R. S. Ramon, S. Gaillard, S. P. Nolan, J. Organomet. Chem. 2011, 696, 7–11.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.