Polyoxometalates as Inhibitors of the Aggregation of Amyloid β Peptides Associated with Alzheimer’s Disease†
Jie Geng
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorMeng Li
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorProf. Jinsong Ren
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorProf. Enbo Wang
Key Laboratory of Polyoxometalate Science of the Ministry of Education, Department of Chemistry, Northeast Normal University, Changchun, Jilin 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Xiaogang Qu
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)Search for more papers by this authorJie Geng
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorMeng Li
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorProf. Jinsong Ren
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Search for more papers by this authorProf. Enbo Wang
Key Laboratory of Polyoxometalate Science of the Ministry of Education, Department of Chemistry, Northeast Normal University, Changchun, Jilin 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Xiaogang Qu
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)
Division of Biological Inorganic Chemistry, State Key Laboratory of Rare Earth Resource Utilization, Laboratory of Chemical Biology, Changchun Institute of Applied Chemistry, Graduate School of the Chinese Academy of Sciences, Changchun, Jilin 130022 (China)Search for more papers by this authorWe acknowledge financial support through the 973 Project (2011CB936004), by the NSFC (20831003, 90813001, 20833006, 90913007, 20903086), and by the Chinese Academy of Sciences.
Graphical Abstract
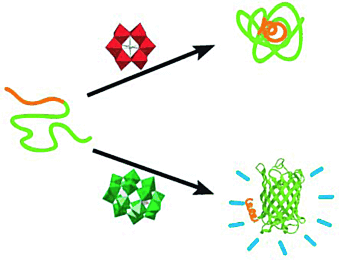
Anorganische Amyloidinhibitoren: Vier Arten von Polyoxometallaten (POMs) erwiesen sich als effiziente Inhibitoren der Amyloidbildung durch Amyloid-β-Peptide (Aβ; siehe Bild: anders als das obere POM (rot) inhibierte das untere (dunkelgrün) die Amyloidbildung). Die Inhibitionsselektivität der POMs beruht auf größenspezifischen elektrostatischen Wechselwirkungen zwischen den POMs und Aβ über die Bindung an die positiv geladene His13–Lys16-Clusterregion von Aβ.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201007067_sm_miscellaneous_information.pdf179.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. S. Kosik, Science 1992, 256, 780–783.
- 2G. Yamin, K. Ono, M. Inayathullah, D. B. Teplow, Curr. Pharm. Des. 2008, 14, 3231–3246.
- 3A. Frydman-Marom, M. Rechter, I. Shefler, Y. Bram, D. E. Shalev, E. Gazit, Angew. Chem. 2009, 121, 2015–2020; Angew. Chem. Int. Ed. 2009, 48, 1981–1986.
- 4T. Takahashi, H. Mihara, Acc. Chem. Res. 2008, 41, 1309–1318.
- 5C. Soto, E. M. Sigurdsson, L. Morelli, R. A. Kumar, E. M. Castano, B. Frangione, Nat. Med. 1998, 4, 822–826.
- 6D. E. Ehrnhoefer, J. Bieschke, A. Boeddrich, M. Herbst, L. Masino, R. Lurz, S. Engemann, A. Pastore, E. E. Wanker, Nat. Struct. Mol. Biol. 2008, 15, 558–566.
- 7F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy, G. M. Cole, J. Biol. Chem. 2005, 280, 5892–5901.
- 8C. Orvig, M. J. Abrams, Chem. Rev. 1999, 99, 2201–2204.
- 9K. J. Barnham, V. B. Kenche, G. D. Ciccotosto, D. P. Smith, D. J. Tew, X. Liu, K. Perez, G. A. Cranston, T. J. Johanssen, I. Volitakis, A. I. Bush, C. L. Masters, A. R. White, J. P. Smith, R. A. Cherny, R. Cappai, Proc. Natl. Acad. Sci. USA 2008, 105, 6813–6818.
- 10J. E. Kim, M. Lee, Biochem. Biophys. Res. Commun. 2003, 303, 576–579.
- 11
- 11aR. Prudent, C. F. Sautel, C. Cochet, Biochim. Biophys. Acta Proteins Proteomics 2010, 1804, 493–498;
- 11bH. Yanagie, A. Ogata, S. Mitsui, T. Hisa, T. Yamase, M. Eriguchi, Biomed. Pharmacother. 2006, 60, 349–352;
- 11cB. Hasenknopf, Front. Biosci. 2005, 10, 275–287;
- 11dJ. T. Rhule, C. L. Hill, D. A. Judd, R. F. Schinazi, Chem. Rev. 1998, 98, 327–358.
- 12D. A. Judd, J. H. Nettles, N. Nevins, J. P. Snyder, D. C. Liotta, J. Tang, J. Ermolieff, R. F. Schinazi, C. L. Hill, J. Am. Chem. Soc. 2001, 123, 886–897.
- 13S. G. Sarafianos, U. Kortz, M. T. Pope, M. J. Modak, Biochem. J. 1996, 319(Pt 2), 619–626.
- 14A. Seko, T. Yamase, K. Yamashita, J. Inorg. Biochem. 2009, 103, 1061–1066.
- 15R. Prudent, V. Moucadel, B. Laudet, C. Barette, L. Lafanechère, B. Hasenknopf, J. Li, S. Bareyt, E. Lacote, S. Thorimbert, M. Malacria, P. Gouzerh, C. Cochet, Chem. Biol. 2008, 15, 683–692.
- 16Q. Wu, J. Wang, L. Zhang, A. Hong, J. Ren, Angew. Chem. 2005, 117, 4116–4120; Angew. Chem. Int. Ed. 2005, 44, 4048–4052.
- 17W. Kim, Y. Kim, J. Min, D. J. Kim, Y. T. Chang, M. H. Hecht, ACS Chem. Biol. 2006, 1, 461–469.
- 18H. LeVine III, Protein Sci. 1993, 2, 404–410.
- 19J. Geng, H. J. Yu, J. Ren, X. Qu, Electrochem. Commun. 2008, 10, 1797–1800.
- 20H. Yu, J. Ren, X. Qu, ChemBioChem 2008, 9, 879–882.
- 21H. LeVine III, Arch. Biochem. Biophys. 2002, 404, 106–115.
- 22A. D. Ferrão-Gonzales, B. K. Robbs, V. H. Moreau, A. Ferreira, L. Juliano, A. P. Valente, F. C. L. Almeida, J. L. Silva, D. Foguel, J. Biol. Chem. 2005, 280, 34747–34754.
- 23J. R. Lakowicz, G. Weber, Biochemistry 1973, 12, 4161–4170.
- 24H. Zhu, J. Yu, M. S. Kindy, Mol. Med. 2001, 7, 517–522.
- 25J. McLaurin, T. Franklin, X. Zhang, J. Deng, P. E. Fraser, Eur. J. Biochem. 1999, 266, 1101–1110.
- 26M. M. Pallitto, J. Ghanta, P. Heinzelman, L. L. Kiessling, R. M. Murphy, Biochemistry 1999, 38, 3570–3578.
- 27M. A. Findeis, G. M. Musso, C. C. Arico-Muendel, H. W. Benjamin, A. M. Hundal, J.-J. Lee, J. Chin, M. Kelley, J. Wakefield, N. J. Hayward, S. M. Molineaux, Biochemistry 1999, 38, 6791–6800.
- 28C. Cabaleiro-Lago, F. Quinlan-Pluck, I. Lynch, S. Lindman, A. M. Minogue, E. Thulin, D. M. Walsh, K. A. Dawson, S. Linse, J. Am. Chem. Soc. 2008, 130, 15437–15443.
- 29C. E. Müller, J. Iqbal, Y. Baqi, H. Zimmermann, A. Rollich, H. Stephan, Bioorg. Med. Chem. Lett. 2006, 16, 5943–5947.
- 30G. Zhang, B. Keita, C. T. Craescu, S. Miron, P. de Oliveira, L. Nadjo, Biomacromolecules 2008, 9, 812–817.
- 31H. Wille, M. Shanmugam, M. Murugesu, J. Ollesch, G. Stubbs, J. R. Long, J. G. Safar, S. B. Prusiner, Proc. Natl. Acad. Sci. USA 2009, 106, 3740–3745.
- 32H. Yu, J. Ren, X. Qu, Biophys. J. 2007, 92, 185–191.
- 33J. Geng, C. Zhao, J. Ren, X. Qu, Chem. Commun. 2010, 46, 7187–7189.
- 34J. Geng, K. Qu, J. Ren, X. Qu, Mol. Biosyst. 2010, 6, 2389–2391.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.