Mechanistic Complexity in Organo–SOMO Activation†
James J. Devery III
Department of Chemistry, Lehigh University, Bethlehem, PA 18015 (USA), Fax: (+1) 610-758-6536 http://www.lehigh.edu/∼rof2/
Search for more papers by this authorJay C. Conrad
Merck Center for Catalysis, Princeton University, Princeton, NJ 08544 (USA)
Search for more papers by this authorDavid W. C. MacMillan Prof.
Merck Center for Catalysis, Princeton University, Princeton, NJ 08544 (USA)
Search for more papers by this authorRobert A. Flowers II Prof.
Department of Chemistry, Lehigh University, Bethlehem, PA 18015 (USA), Fax: (+1) 610-758-6536 http://www.lehigh.edu/∼rof2/
Search for more papers by this authorJames J. Devery III
Department of Chemistry, Lehigh University, Bethlehem, PA 18015 (USA), Fax: (+1) 610-758-6536 http://www.lehigh.edu/∼rof2/
Search for more papers by this authorJay C. Conrad
Merck Center for Catalysis, Princeton University, Princeton, NJ 08544 (USA)
Search for more papers by this authorDavid W. C. MacMillan Prof.
Merck Center for Catalysis, Princeton University, Princeton, NJ 08544 (USA)
Search for more papers by this authorRobert A. Flowers II Prof.
Department of Chemistry, Lehigh University, Bethlehem, PA 18015 (USA), Fax: (+1) 610-758-6536 http://www.lehigh.edu/∼rof2/
Search for more papers by this authorR.A.F. and D.W.C.M. are grateful to the National Institutes of Health (1R15M075960-01 and R01-GM093213-01) for support of this work.
Graphical Abstract
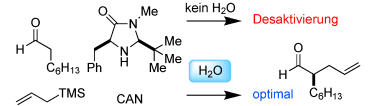
Nasse Chemie: „Organo-SOMO-Aktivierung“ ist ein komplizierter Prozess. Der Katalysator ist ohne H2O desaktiviert, und seine Konzentration wird durch 2 Äquiv. H2O konstant gehalten. Die kinetische Rolle von Cerammoniumnitrat (CAN) wird durch Phasentransfer maskiert, und seine begrenzte Löslichkeit wird durch zugegebenes H2O verbessert. Mechanistischen Studien zufolge erhöht die sorgfältige Zugabe von H2O zu getrockneten Reagentien die Reaktionseffizienz. TMS=Trimethylsilyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201001673_sm_miscellaneous_information.pdf3.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. D. Beeson, A. Mastracchio, J.-B. Hong, K. Ashton, D. W. C. MacMillan, Science 2007, 316, 582–585;
- 1bH.-Y. Jang, J.-B. Hong, D. W. C. MacMillan, J. Am. Chem. Soc. 2007, 129, 7004–7005;
- 1cH. Kim, D. W. C. MacMillan, J. Am. Chem. Soc. 2008, 130, 398–399;
- 1dT. H. Graham, C. M. Jones, N. T. Jui, D. W. C. MacMillan, J. Am. Chem. Soc. 2008, 130, 16494–16495;
- 1eM. Amatore, T. D. Beeson, S. P. Brown, D. W. C. MacMillan, Angew. Chem. 2009, 121, 5223–5226; Angew. Chem. Int. Ed. 2009, 48, 5121–5124;
- 1fS. Rendler, D. W. C. MacMillan, J. Am. Chem. Soc. 2010, 132, 5027–5029.
- 2
- 2aK. C. Nicolaou, R. Reingruber, D. Sarlah, S. Bräse, J. Am. Chem. Soc. 2009, 131, 2086–2087;
- 2bJ. C. Conrad, J. Kong, B. N. Laforteza, D. W. C. MacMillan, J. Am. Chem. Soc. 2009, 131, 11640–11641;
- 2cJ. M. Um, O. Gutierrez, F. Schoenebeck, K. N. Houk, D. W. C. MacMillan, J. Am. Chem. Soc. 2010, 132, 6001–6005.
- 3
- 3aT. Chiba, M. Okimoto, H. Nagai, Y. Takata, J. Org. Chem. 1979, 44, 3519–3523;
- 3bK. Kaneda, T. Itoh, N. Kii, K. Jitsukawa, S. Teranishi, J. Mol. Cat. 1982, 15, 349–365.
- 4
- 4aK. Watanabe, J. R. Mottl, J. Chem. Phys. 1957, 26, 1733–1734;
- 4bA. Adenier, M. M. Chemimi, I. Gallardo, J. Pinson, N. Vila, Langmuir 2004, 20, 8243–8253.
- 5
- 5aD. G. Blackmond, Angew. Chem. 2005, 117, 4374–4393;
10.1002/ange.200462544 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4302–4320;
- 5bJ. S. Mathew, M. Klussmann, H. Iwamura, F. Valera, A. Futran, E. A. C. Emanuelsson, D. G. Blackmond, J. Org. Chem. 2006, 71, 4711–4722.
- 6M. Gruttadauria, F. Giacalone, R. Noto, Adv. Synth. Catal. 2009, 351, 33–57.
- 7K. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243–4244.
- 8D. E. Ward, V. Jheengut, Tetrahedron Lett. 2004, 45, 8347–8350.
- 9
- 9aA. I. Nyberg, A. Usano, P. M. Pihko, Synlett 2004, 1891–1897;
- 9bP. M. Pihko, K. M. Laurikainen, A. Usano, A. Nyberg, J. A. Kaavi, Tetrahedron 2006, 62, 317–328.
- 10
- 10aN. Zotova, A. Franzke, A. Armstrong, D. G. Blackmond, J. Am. Chem. Soc. 2007, 129, 15100–15101;
- 10bN. Zotova, L. J. Broadbelt, A. Armstrong, D. G. Blackmond, Bioorg. Med. Chem. Lett. 2009, 19, 3934–3937.
- 11Plots used to determine the rate orders of the components are found in the Supporting Information.
- 12J. Jiao, Y. Zhang, J. J. Devery III, L. Xu, J. Deng, R. A. Flowers II, J. Org. Chem. 2007, 72, 5486–5492. The nucleophile can be either nitrate, water, or carbonate.
- 13The CAN test is a chemical test used to determine the presence of OH moieties. Coordination to CeIV is indicated by a red color; see R. L. Shriner, C. K. F. Hermann, T. C. Morril, D. Y. Curtin, R. C. Fuson, The Systematic Identification of Organic Compounds, 8th ed., Wiley, Hoboken, NJ, 2004, pp. 265–268.
- 14M. Wiesner, G. Upert, G. Angelici, H. Wennemers, J. Am. Chem. Soc. 2010, 132, 6–7.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.