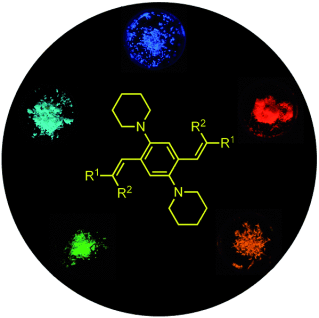
1,4-Bis(alkenyl)-2,5-dipiperidinobenzenes: Minimal Fluorophores Exhibiting Highly Efficient Emission in the Solid State†
Masaki Shimizu Prof. Dr.
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorYouhei Takeda
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorMasahiro Higashi
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorTamejiro Hiyama Prof. Dr.
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorMasaki Shimizu Prof. Dr.
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorYouhei Takeda
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorMasahiro Higashi
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorTamejiro Hiyama Prof. Dr.
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2445, http://npc05.kuic.kyoto-u.ac.jp/e-index.htm
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Creative Scientific Research (No. 16GS0209) from the Ministry of Education, Culture, Sports, Science and Technology (Japan).
Abstract
Minimale Anforderung: Kristalle und dünne Filme von 1,4-Bis(alkenyl)-2,5-dipiperidinobenzolen, die lediglich einen Benzolring als aromatische Komponente enthalten, emittieren sichtbares Licht mit hervorragenden Quantenausbeuten, wenn sie mit UV-Licht bestrahlt werden. Mit diesen Verbindungen dotierte dünne Polystyrolfilme fluoreszieren ebenfalls kräftig. Durch Variation der Alkenylgruppen werden Farben im Bereich zwischen Blau und Rot erzielt.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200900963_sm_miscellaneous_information.pdf3.4 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Highly Efficient OLEDs with Phosphorescent Materials (Ed.: ), Wiley-VCH, Weinheim, 2008;
- 1b Organic Light-Emitting Devices. Synthesis Properties and Applications (Eds.: ), Wiley-VCH, Weinheim, 2006;
- 1cR. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Brédas, M. Lögdlund, W. R. Salaneck, Nature 1999, 397, 121–128.
- 2
- 2aI. D. W. Samuel, G. A. Turnbull, Chem. Rev. 2007, 107, 1272–1295;
- 2bI. D. W. Samuel, G. A. Turnbull, Mater. Today 2004, 7, 28–35;
- 2cU. Scherf, S. Riechel, U. Lemmer, R. F. Mahrt, Curr. Opin. Solid State Mater. Sci. 2001, 5, 143–154;
- 2dM. D. McGehee, A. J. Heeger, Adv. Mater. 2000, 12, 1655–1668;
- 2eG. Kranzelbinder, G. Leising, Rep. Prog. Phys. 2000, 63, 729–762;
- 2fN. Tessler, Adv. Mater. 1999, 11, 363–370;
- 2gV. G. Kozlov, S. R. Forrest, Curr. Opin. Solid State Mater. Sci. 1999, 4, 203–208.
- 3Reviews on fluorescent sensors:
- 3aS. W. Thomas Iii, G. D. Joly, T. M. Swager, Chem. Rev. 2007, 107, 1339–1386;
- 3bL. Basabe-Desmonts, D. N. Reinhoudt, M. Crego-Calama, Chem. Soc. Rev. 2007, 36, 993–1017;
- 3cO. S. Wolfbeis, J. Mater. Chem. 2005, 15, 2657–2669;
- 3dJ. F. Callan, A. P. de Silva, D. C. Magri, Tetrahedron 2005, 61, 8551–8588;
- 3eR. Martínez-Máñez, F. Sancenon, Chem. Rev. 2003, 103, 4419–4476;
- 3fA. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515–1566. Selected examples of solid-state fluorescent sensors:
- 3gS. Sreejith, K. P. Divya, A. Ajayaghosh, Chem. Commun. 2008, 2903–2905;
- 3hT. J. Dale, J. Rebek, J. Am. Chem. Soc. 2006, 128, 4500–4501;
- 3iS. W. Zhang, T. M. Swager, J. Am. Chem. Soc. 2003, 125, 3420–3421;
- 3jJ. S. Yang, T. M. Swager, J. Am. Chem. Soc. 1998, 120, 11864–11873;
- 3kJ. S. Yang, T. M. Swager, J. Am. Chem. Soc. 1998, 120, 5321–5322.
- 4
- 4aT. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker, J. Salbeck, Chem. Rev. 2007, 107, 1011–1065;
- 4bP. L. Burn, S. C. Lo, I. D. W. Samuel, Adv. Mater. 2007, 19, 1675–1688;
- 4cI. F. Perepichka, D. F. Perepichka, H. Meng, F. Wudl, Adv. Mater. 2005, 17, 2281–2305;
- 4dL. Akcelrud, Prog. Polym. Sci. 2003, 28, 875–962;
- 4eU. Mitschke, P. Bäuerle, J. Mater. Chem. 2000, 10, 1471–1507;
- 4fD. Y. Kim, H. N. Cho, C. Y. Kim, Prog. Polym. Sci. 2000, 25, 1089–1139;
- 4gA. Kraft, A. C. Grimsdale, A. B. Holmes, Angew. Chem. 1998, 110, 416–443;
10.1002/(SICI)1521-3757(19980216)110:4<416::AID-ANGE416>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1998, 37, 402–428.10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 5
- 5aW. Y. Lai, R. Xia, Q. Y. He, P. A. Levermore, W. Huang, D. D. C. Bradley, Adv. Mater. 2009, 21, 355–360;
- 5bM. Shimizu, K. Mochida, T. Hiyama, Angew. Chem. 2008, 120, 9906–9910; Angew. Chem. Int. Ed. 2008, 47, 9760–9764;
- 5cJ. Huang, X. Qiao, Y. Xia, X. Zhu, D. Ma, Y. Cao, J. Roncali, Adv. Mater. 2008, 20, 4172–4175;
- 5dM. Y. Lai, C. H. Chen, W. S. Huang, J. T. Lin, T. H. Ke, L. Y. Chen, M. H. Tsai, C. C. Wu, Angew. Chem. 2008, 120, 591–595; Angew. Chem. Int. Ed. 2008, 47, 581–585;
- 5eE. Ishow, A. Brosseau, G. Clavier, K. Nakatani, P. Tauc, C. Fiorini-Debuisschert, S. Neveu, O. Sandre, A. Léaustic, Chem. Mater. 2008, 20, 6597–6599;
- 5fY. T. Lee, C. L. Chiang, C. T. Chen, Chem. Commun. 2008, 217–219;
- 5gC. Kitamura, C. Matsumoto, N. Kawatsuki, A. Yoneda, K. Asada, T. Kobayashi, H. Naito, Bull. Chem. Soc. Jpn. 2008, 81, 754–756;
- 5hZ. Chen, J. Bouffard, S. E. Kooi, T. M. Swager, Macromolecules 2008, 41, 6672–6676;
- 5iA. Wakamiya, K. Mori, S. Yamaguchi, Angew. Chem. 2007, 119, 4351–4354; Angew. Chem. Int. Ed. 2007, 46, 4273–4276;
- 5jJ. Luo, Y. Zhou, Z. Q. Niu, Q. F. Zhou, Y. Ma, J. Pei, J. Am. Chem. Soc. 2007, 129, 11314–11315;
- 5kZ. Xie, H. Wang, F. Li, W. Xie, L. Liu, B. Yang, L. Ye, Y. Ma, Cryst. Growth Des. 2007, 7, 2512–2516;
- 5lY. Li, F. Li, H. Zhang, Z. Xie, W. Xie, H. Xu, B. Li, F. Shen, L. Ye, M. Hanif, D. Ma, Y. Ma, Chem. Commun. 2007, 231–233;
- 5mC. H. Zhao, A. Wakamiya, Y. Inukai, S. Yamaguchi, J. Am. Chem. Soc. 2006, 128, 15934–15935;
- 5nA. Hayer, V. de Halleux, A. Köhler, A. El-Garoughy, E. W. Meijer, J. Barbera, J. Tant, J. Levin, M. Lehmann, J. Gierschner, J. Cornil, Y. H. Geerts, J. Phys. Chem. B 2006, 110, 7653–7659;
- 5oY. Kim, J. Bouffard, S. E. Kooi, T. M. Swager, J. Am. Chem. Soc. 2005, 127, 13726–13731;
- 5pS. H. Lee, B. B. Jang, Z. H. Kafafi, J. Am. Chem. Soc. 2005, 127, 9071–9078;
- 5qK. L. Chan, M. J. McKiernan, C. R. Towns, A. B. Holmes, J. Am. Chem. Soc. 2005, 127, 7662–7663;
- 5rG. Yui, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng, Z. Shuai, B. Tang, D. Zhu, W. Fang, Y. Luo, J. Am. Chem. Soc. 2005, 127, 6335–6346;
- 5sT. C. Chao, Y. T. Lin, C. Y. Yang, T. S. Hung, H. C. Chou, C. C. Wu, K. T. Wong, Adv. Mater. 2005, 17, 992–996;
- 5tK. T. Wong, Y. Y. Chien, R. T. Chen, C. F. Wang, Y. T. Lin, H. H. Chiang, P. Y. Hsieh, C. C. Wu, C. H. Chou, Y. O. Su, G. H. Lee, S. M. Peng, J. Am. Chem. Soc. 2002, 124, 11576–11577;
- 5uM. Ariu, D. G. Lidzey, M. Sims, A. J. Cadby, P. A. Lane, D. D. C. Bradley, J. Phys. Condens. Matter 2002, 14, 9975–9986.
- 6Pyromellitic diimides were recently reported to act as minimal cores for n-channel transistor materials with high mobility. Q. Zheng, J. Huang, A. Sarjeant, H. E. Katz, J. Am. Chem. Soc. 2008, 130, 14410–14411.
- 7Unpublished results. Details will be reported in due course.
- 8Cyanovinyl-substituted dimethoxybenzenes were reported to exhibit fluorescence in ethanol with emission maxima ranging from 500 to 580 nm;
- 8aK. Y. Chu, J. Griffiths, D. Ward, J. Chem. Res. Synop. 1981, 300–301;
- 8bK. Y. Chu, J. Griffiths, D. Ward, J. Chem. Res. Miniprint 1981, 3701–3721.
- 9Z. Xie, B. Yang, L. Liu, M. Li, D. Lin, Y. Ma, G. Cheng, S. Liu, J. Phys. Org. Chem. 2005, 18, 962–973.
- 10
- 10aT. Kobayashi, T. Eda, O. Tamura, H. Ishibashi, J. Org. Chem. 2002, 67, 3156–3159;
- 10bJ. P. Wolfe, S. L. Buchwald, J. Org. Chem. 2000, 65, 1144–1157. See the Supporting Information.
- 11UV irradiation of 1–5 both in cyclohexane and in the crystal with a 400 W high-pressure mercury lamp at room temperature for 1 h resulted in no change of their absorption and fluorescence spectra.
- 12CCDC 719745 (3) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13The molecular structure was optimized by DFT methods at the B3LYP/6-31G(d) level with the crystal structure as the initial geometry.
- 14For the preparation of 1, 2, 4, and 5, see the Supporting Information. These benzenes are also soluble in organic solvents such as CH2Cl2, CHCl3, THF, and toluene.
- 15Absorption and fluorescence spectra of 1–5 in cyclohexane and fluorescence spectra in thin neat films and in polystyrene films are shown in the Supporting Information.
- 16
- 16aC. T. Chen, Chem. Mater. 2004, 16, 4389–4400;
- 16bY. H. Song, S. J. Yeh, C. T. Chen, Y. Chi, C. S. Liu, J. K. Yu, Y. H. Hu, P. T. Chou, S. M. Peng, G. H. Lee, Adv. Funct. Mater. 2004, 14, 1221–1226;
- 16cC. L. Chiang, M. F. Wu, D. C. Dai, Y. S. Wen, J. K. Wang, C. T. Chen, Adv. Funct. Mater. 2005, 15, 231–238;
- 16dY. L. Tung, S. W. Lee, Y. Chi, Y. T. Tao, C. H. Chien, Y. M. Cheng, P. T. Chou, S. M. Peng, C. S. Liu, J. Mater. Chem. 2005, 15, 460–464;
- 16eRef. [5i];
- 16fRef. [5f];
- 16gA. Wakamiya, N. Sugita, S. Yamaguchi, Chem. Lett. 2008, 37, 1094–1095.
- 17
- 17aJ. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, K. Hoi Sing, X. Zhan, Y. Liu, D. Zhu, T. Ben Zhong, Chem. Commun. 2001, 1740–1741;
- 17bK. Itami, Y. Ohashi, J. I. Yoshida, J. Org. Chem. 2005, 70, 2778–2792;
- 17cH. Tong, Y. Hong, Y. Dong, M. Häussler, J. W. Y. Lam, Z. Li, Z. Guo, Z. Guo, B. Z. Tang, Chem. Commun. 2006, 3705–3707;
- 17dS. Kim, Q. Zheng, G. S. He, D. J. Bharali, H. E. Pudavar, A. Baev, P. N. Prasad, Adv. Funct. Mater. 2006, 16, 2317–2323;
- 17eZ. Ning, Z. Chen, Q. Zhang, Y. Yan, S. Qian, Y. Cao, H. Tian, Adv. Funct. Mater. 2007, 17, 3799–3807;
- 17fY. Dong, J. W. Y. Lam, A. Qin, J. Sun, J. Liu, Z. Li, J. Sun, H. H. Y. Sung, I. D. Williams, H. S. Kwok, B. Z. Tang, Chem. Commun. 2007, 3255–3257. Fluorescence spectra of 3 measured in a mixture of CH3CN/H2O are shown in the Supporting Information.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.