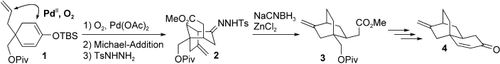Formal Total Synthesis of Platencin†
Georgy N. Varseev Dipl.-Chem.
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+49) 7071-295-137, http://www.uni-tuebingen.de/uni/com/welcome.htm
Search for more papers by this authorMartin E. Maier Prof. Dr.
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+49) 7071-295-137, http://www.uni-tuebingen.de/uni/com/welcome.htm
Search for more papers by this authorGeorgy N. Varseev Dipl.-Chem.
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+49) 7071-295-137, http://www.uni-tuebingen.de/uni/com/welcome.htm
Search for more papers by this authorMartin E. Maier Prof. Dr.
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany), Fax: (+49) 7071-295-137, http://www.uni-tuebingen.de/uni/com/welcome.htm
Search for more papers by this authorFinancial support by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie is gratefully acknowledged. We thank Graeme Nicholson for HRMS measurements and analytical GC on a chiral phase.
Abstract
Der richtige Bicyclus: Die effiziente sauerstoffvermittelte palladiumkatalysierte Cycloalkenylierung von 1 zu einem Bicyclo[3.2.1]octan und die desoxygenierende Umlagerung des Tosylhydrazons 2 zum Bicyclo[2.2.2]octan 3 sind wesentliche Schritte der Synthese der Kernstruktur 4 von Platencin, deren Gesamtausbeute nach 13 Stufen ausgehend von einer käuflich erhältlichen Verbindung 17.5 % betrug. Ts=p-Toluolsulfonyl, TBS=tert-Butyldimethylsilyl, Piv=Pivaloyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200900447_sm_miscellaneous_information.pdf1.1 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aF. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich, Angew. Chem. 2006, 118, 5194–5254;
10.1002/ange.200600350 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5072–5129;
- 1bK. C. Nicolaou, J. S. Chen, D. J. Edmonds, A. A. Estrada, Angew. Chem. 2009, 121, 670–732;
10.1002/ange.200801695 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 660–719.
- 2B. Bister, D. Bischoff, M. Ströbele, J. Riedlinger, A. Reicke, A. T. Bull, H. Zähner, H.-P. Fiedler, R. D. Süssmuth, Angew. Chem. 2004, 116, 2628–2630;
10.1002/ange.200353160 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2574–2576.
- 3
- 3aJ. Wang, S. Kodali, S. H. Lee, A. Galgoci, R. Painter, K. Dorso, F. Racine, M. Motyl, L. Hernandez, E. Tinney, S. L. Colletti, K. Herath, R. Cummings, O. Salazar, I. Gonzalez, A. Basilio, F. Vicente, O. Genilloud, F. Pelaez, H. Jayasuriya, K. Young, D. F. Cully, S. B. Singh, Proc. Natl. Acad. Sci. USA 2007, 104, 7612–7616;
- 3bH. Jayasuriya, K. B. Herath, C. Zhang, D. L. Zink, A. Basilio, O. Genilloud, M. T. Diez, F. Vicente, I. Gonzalez, O. Salazar, F. Pelaez, R. Cummings, S. Ha, J. Wang, S. B. Singh, Angew. Chem. 2007, 119, 4768–4772;
10.1002/ange.200701058 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4684–4688.
- 4J. Wang, S. M. Soisson, K. Young, W. Shoop, S. Kodali, A. Galgoci, R. Painter, G. Parthasarathy, Y. S. Tang, R. Cummings, S. Ha, K. Dorso, M. Motyl, H. Jayasuriya, J. Ondeyka, K. Herath, C. Zhang, L. Hernandez, J. Allocco, A. Basilio, J. R. Tormo, O. Genilloud, F. Vicente, F. Pelaez, L. Colwell, S. H. Lee, B. Michael, T. Felcetto, C. Gill, L. L. Silver, J. D. Hermes, K. Bartizal, J. Barrett, D. Schmatz, J. W. Becker, D. Cully, S. B. Singh, Nature 2006, 441, 358–361.
- 5P. Johansson, B. Wiltschi, P. Kumari, B. Kessler, C. Vonrhein, J. Vonck, D. Oesterhelt, M. Grininger, Proc. Natl. Acad. Sci. USA 2008, 105, 12803–12808.
- 6K. B. Herath, A. B. Attygalle, S. B. Singh, J. Am. Chem. Soc. 2007, 129, 15422–15423.
- 7K. Herath, A. B. Attygalle, S. B. Singh, Tetrahedron Lett. 2008, 49, 5755–5758.
- 8
- 8aA. Roy, F. G. Roberts, P. R. Wilderman, K. Zhou, R. J. Peters, R. M. Coates, J. Am. Chem. Soc. 2007, 129, 12453–12460, and references therein;
- 8bM. Xu, P. R. Wilderman, R. J. Peters, Proc. Natl. Acad. Sci. USA 2007, 104, 7397–7401.
- 9For a review, see: K. Tiefenbacher, J. Mulzer, Angew. Chem. 2008, 120, 2582–2590;
10.1002/ange.200705303 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2548–2555.
- 10For some recent synthetic studies related to platensimycin, see:
- 10aK. C. Nicolaou, D. Pappo, K. Y. Tsang, R. Gibe, D. Y. K. Chen, Angew. Chem. 2008, 120, 958–960;
10.1002/ange.200705080 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 944–946;
- 10bC. H. Kim, K. P. Jang, S. Y. Choi, Y. K. Chung, E. Lee, Angew. Chem. 2008, 120, 4073–4075; Angew. Chem. Int. Ed. 2008, 47, 4009–4011;
- 10cK. C. Nicolaou, A. F. Stepan, T. Lister, A. Li, A. Montero, G. S. Tria, C. I. Turner, Y. Tang, J. Wang, R. M. Denton, D. J. Edmonds, J. Am. Chem. Soc. 2008, 130, 13110–13119;
- 10dJ.-i. Matsuo, K. Takeuchi, H. Ishibashi, Org. Lett. 2008, 10, 4049–4052.
- 11
- 11aK. C. Nicolaou, G. S. Tria, D. J. Edmonds, Angew. Chem. 2008, 120, 1804–1807;
10.1002/ange.200800066 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1780–1783;
- 11bJ. Hayashida, V. H. Rawal, Angew. Chem. 2008, 120, 4445–4448; Angew. Chem. Int. Ed. 2008, 47, 4373–4376;
- 11cS. Y. Yun, J.-C. Zheng, D. Lee, Angew. Chem. 2008, 120, 6297–6299; Angew. Chem. Int. Ed. 2008, 47, 6201–6203;
- 11dK. Tiefenbacher, J. Mulzer, Angew. Chem. 2008, 120, 6294–6295;
10.1002/ange.200801441 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6199–6200;
- 11eD. C. J. Waalboer, M. C. Schaapman, F. L. van Delft, F. P. J. T. Rutjes, Angew. Chem. 2008, 120, 6678–6680;
10.1002/ange.200802912 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6576–6578;
- 11fK. C. Nicolaou, Q.-Y. Toh, D. Y. K. Chen, J. Am. Chem. Soc. 2008, 130, 11292–11293; K. C. Nicolaou, Q.-Y. Toh, D. Y. K. Chen, J. Am. Chem. Soc. 2008, 130, 14016;
- 11gK. A. B. Austin, M. G. Banwell, A. C. Willis, Org. Lett. 2008, 10, 4465–4468.
- 12See, for example:
- 12aS. A. Monti, S.-C. Chen, Y.-L. Yang, S.-S. Yuan, O. P. Bourgeois, J. Org. Chem. 1978, 43, 4062–4069;
- 12bD. H. Hua, W. Y. Gung, R. A. Ostrander, F. Takusagawa, J. Org. Chem. 1987, 52, 2509–2517;
- 12cD. Kim, P. J. Shim, J. Lee, C. W. Park, S. W. Hong, S. Kim, J. Org. Chem. 2000, 65, 4864–4870.
- 13For a review about the synthesis of [3.2.1] systems, see: M.-H. Filippini, J. Rodriguez, Chem. Rev. 1999, 99, 27–76.
- 14
- 14aM. Toyota, T. Wada, K. Fukumoto, M. Ihara, J. Am. Chem. Soc. 1998, 120, 4916–4925;
- 14bM. Toyota, T. Asano, M. Ihara, Org. Lett. 2005, 7, 3929–3932.
- 15For reviews, see:
- 15aD. C. Nonhebel, Chem. Soc. Rev. 1993, 22, 347–359;
- 15bP. Dowd, W. Zhang, Chem. Rev. 1993, 93, 2091–2115.
- 16G. N. Varseev, Tübinger-Göttinger Gespräche zur Chemie von Mikroorganismen, Zellingen-Retzbach, September 19, 2007.
- 17M. Toyota, T. Wada, Y. Nishikawa, K. Yanai, K. Fukumoto, C. Kabuto, Tetrahedron 1995, 51, 6927–6940.
- 18G. Stork, R. L. Danheiser, J. Org. Chem. 1973, 38, 1775–1776.
- 19
- 19aR. Hara, T. Furukawa, H. Kashima, H. Kusama, Y. Horiguchi, I. Kuwajima, J. Am. Chem. Soc. 1999, 121, 3072–3082;
- 19bS. Yamada, H. Suemune, Chem. Pharm. Bull. 2000, 48, 1171–1175.
- 20Y. Ito, T. Hirao, T. Saegusa, J. Org. Chem. 1978, 43, 1011–1013.
- 21G. N. Varseev, unpublished results.
- 22
- 22aD. C. Behenna, B. M. Stoltz, J. Am. Chem. Soc. 2004, 126, 15044–15045;
- 22bJ. T. Mohr, D. C. Behenna, A. M. Harned, B. M. Stoltz, Angew. Chem. 2005, 117, 7084–7087;
10.1002/ange.200502018 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6924–6927;
- 22cS. R. Levine, M. R. Krout, B. M. Stoltz, Org. Lett. 2009, 11, 289–292;
- 22dK. V. Petrova, J. T. Mohr, B. M. Stoltz, Org. Lett. 2009, 11, 293–295.
- 23For a review, see: J. T. Mohr, B. M. Stoltz, Chem. Asian J. 2007, 2, 1476–1491.
- 24
- 24aK. Narasaka, K. Soai, Y. Aikawa, T. Mukaiyama, Bull. Chem. Soc. Jpn. 1976, 49, 779–783;
- 24bC. H. Heathcock, M. H. Norman, D. E. Uehling, J. Am. Chem. Soc. 1985, 107, 2797–2799.
- 25
- 25aY. Kita, J. Segawa, J. Haruta, H. Yasuda, Y. Tamura, J. Chem. Soc. Perkin Trans. 1 1982, 1099–1104;
- 25bC. H. Heathcock, S. K. Davidsen, K. T. Hug, L. A. Flippin, J. Org. Chem. 1986, 51, 3027–3037;
- 25cA. G. Wenzel, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 12964–12965.
- 26
- 26aD. H. R. Barton, S. W. McCombie, J. Chem. Soc. Perkin Trans. 1 1975, 1574–1585;
- 26bB. M. Trost, N. Cramer, H. Bernsmann, J. Am. Chem. Soc. 2007, 129, 3086–3087.
- 27H. S. Park, H. Y. Lee, Y. H. Kim, Org. Lett. 2005, 7, 3187–3190.
- 28For reviews, see:
- 28aB. M. Trost, J. Org. Chem. 2004, 69, 5813–5837;
- 28bB. M. Trost, M. R. Machacek, A. Aponick, Acc. Chem. Res. 2006, 39, 747–760;
- 28cB. M. Trost, C. Jiang, Synthesis 2006, 369–396.
- 29B. M. Trost, R. N. Bream, J. Xu, Angew. Chem. 2006, 118, 3181–3184;
10.1002/ange.200504421 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3109–3112.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.