Reactions of a Copper(II) Superoxo Complex Lead to CH and OH Substrate Oxygenation: Modeling Copper-Monooxygenase CH Hydroxylation†
Debabrata Maiti
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorDong-Heon Lee Prof. Dr.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Current addresses: Department of Chemistry and Research Institute of Physics and Chemistry (RINPAC), Chonbuk National University, Jeonju 561-756, Korea
Search for more papers by this authorKatya Gaoutchenova Dr.
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35032 Marburg, Germany
Search for more papers by this authorChristian Würtele
Institut für Anorganische und Analytische Chemie, Justus-Liebig-Universität Gießen, Heinrich-Buff-Ring 58, 35392 Gießen, Germany
Search for more papers by this authorMax C. Holthausen Prof. Dr.
Institut für Anorganische und Analytische Chemie, Johann Wolfgang Goethe-Universität Frankfurt, Max-von-Laue Strasse 7, 60438 Frankfurt am Main, Germany
Search for more papers by this authorAmy A. Narducci Sarjeant Dr.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorJörg Sundermeyer Prof. Dr.
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35032 Marburg, Germany
Search for more papers by this authorSiegfried Schindler Prof. Dr.
Institut für Anorganische und Analytische Chemie, Justus-Liebig-Universität Gießen, Heinrich-Buff-Ring 58, 35392 Gießen, Germany
Search for more papers by this authorKenneth D. Karlin Prof.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorDebabrata Maiti
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorDong-Heon Lee Prof. Dr.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Current addresses: Department of Chemistry and Research Institute of Physics and Chemistry (RINPAC), Chonbuk National University, Jeonju 561-756, Korea
Search for more papers by this authorKatya Gaoutchenova Dr.
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35032 Marburg, Germany
Search for more papers by this authorChristian Würtele
Institut für Anorganische und Analytische Chemie, Justus-Liebig-Universität Gießen, Heinrich-Buff-Ring 58, 35392 Gießen, Germany
Search for more papers by this authorMax C. Holthausen Prof. Dr.
Institut für Anorganische und Analytische Chemie, Johann Wolfgang Goethe-Universität Frankfurt, Max-von-Laue Strasse 7, 60438 Frankfurt am Main, Germany
Search for more papers by this authorAmy A. Narducci Sarjeant Dr.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorJörg Sundermeyer Prof. Dr.
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35032 Marburg, Germany
Search for more papers by this authorSiegfried Schindler Prof. Dr.
Institut für Anorganische und Analytische Chemie, Justus-Liebig-Universität Gießen, Heinrich-Buff-Ring 58, 35392 Gießen, Germany
Search for more papers by this authorKenneth D. Karlin Prof.
Department of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA, Fax: (+1) 410-516-7044
Search for more papers by this authorWe are grateful to the NIH (K.D.K., GM28962) and Deutsche Forschungsgemeinschaft (J.S., S.S., and M.C.H.) for research support.
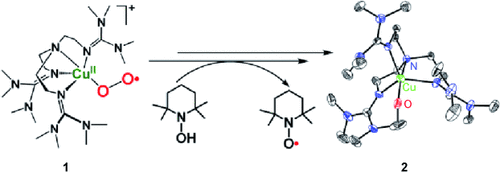
Graphical Abstract
Der Donor macht den Unterschied: Der Disauerstoff-Ligand des einkernigen η1-Superoxo-Kupfer(II)-Komplexes 1 wird durch Wasserstoffatomdonoren aktiviert. Der resultierende Einschub eines O-Atoms aus der O2-Gruppe in die N-gebundene Methylgruppe des Chelatliganden führt zu dem Kupfer(II)-alkoxid 2. Ohne Zusatz des Donors ist die Superoxospezies 1 nicht in der Lage, die beobachtete Hydroxylierung einzugehen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2008/z704389_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. P. Klinman, Chem. Rev. 1996, 96, 2541–2561;
- 1bM. A. McGuirl, D. M. Dooley in Encyclopedia of Inorganic Chemistry, Vol. II, 2nd ed. ), Wiley, Chichester, 2005, pp. 1201–1225;
- 1cJ. P. Klinman, J. Biol. Chem. 2005, 281, 3013–3016;
- 1dP. Chen, E. I. Solomon, Proc. Natl. Acad. Sci. USA 2004, 101, 13105–13110;
- 1eS. Itoh, Curr. Opin. Chem. Biol. 2006, 10, 115–122;
- 1fK. Yoshizawa, N. Kihara, T. Kamachi, Y. Shiota, Inorg. Chem. 2006, 45, 3034–3041;
- 1gA. Crespo, M. A. Marti, A. E. Roitberg, L. M. Amzel, D. A. Estrin, J. Am. Chem. Soc. 2006, 128, 12817–12828.
- 2
- 2aD. Maiti, H. C. Fry, J. S. Woertink, M. A. Vance, E. I. Solomon, K. D. Karlin, J. Am. Chem. Soc. 2007, 129, 264–265;
- 2bD. Maiti, H. R. Lucas, A. A. N. Sarjeant, K. D. Karlin, J. Am. Chem. Soc. 2007, 129, 6998–6999;
- 2cD. Maiti, A. A. Narducci Sarjeant, K. D. Karlin, J. Am. Chem. Soc. 2007, 129, 6720–6721;
- 2dM. Mizuno, K. Honda, J. Cho, H. Furutachi, T. Tosha, T. Matsumoto, S. Fujinami, T. Kitagawa, M. Suzuki, Angew. Chem. 2006, 118, 7065–7068;
10.1002/ange.200602477 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6911–6914;
- 2eT. Fujii, S. Yamaguchi, Y. Funahashi, T. Ozawa, T. Tosha, T. Kitagawa, H. Masuda, Chem. Commun. 2006, 4428–4430;
- 2fS. Yamaguchi, H. Masuda, Sci. Technol. Adv. Mater. 2005, 6, 34–47;
- 2gT. Fujii, A. Naito, S. Yamaguchi, A. Wada, Y. Funahashi, K. Jitsukawa, S. Nagatomo, T. Kitagawa, H. Masuda, Chem. Commun. 2003, 2700–2701.
- 3
- 3aA. Decker, E. I. Solomon, Curr. Opin. Chem. Biol. 2005, 9, 152–163;
- 3bD. Schröder, M. C. Holthausen, H. Schwarz, J. Phys. Chem. B 2004, 108, 14407–14416;
- 3cY. Nakao, K. Hirao, T. Taketsugu, J. Chem. Phys. 2001, 114, 7935–7940.
- 4S. T. Prigge, B. Eipper, R. Mains, L. M. Amzel, Science 2004, 304, 864–867.
- 5
- 5aJ. P. Evans, K. Ahn, J. P. Klinman, J. Biol. Chem. 2003, 278, 49691–49698;
- 5bA. T. Bauman, E. T. Yukl, K. Alkevich, A. L. McCormack, N. J. Blackburn, J. Biol. Chem. 2006, 281, 4190–4198.
- 6
- 6aP. Chen, K. Fujisawa, E. I. Solomon, J. Am. Chem. Soc. 2000, 122, 10177–10193;
- 6bB. Gherman, D. Heppner, W. Tolman, C. Cramer, J. Biol. Inorg. Chem. 2006, 11, 197.
- 7C. Cramer, W. Tolman, Acc. Chem. Res. 2007, 40, 601–608.
- 8
- 8aC. Würtele, E. Gaoutchenova, K. Harms, M. C. Holthausen, J. Sundermeyer, S. Schindler, Angew. Chem. 2006, 118, 3951–3954;
10.1002/ange.200600351 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3867–3869;
- 8bM. Schatz, V. Raab, S. P. Foxon, G. Brehm, S. Schneider, M. Reiher, M. C. Holthausen, J. Sundermeyer, S. Schindler, Angew. Chem. 2004, 116, 4460–4464;
10.1002/ange.200454125 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4360–4363.
- 9See the Supporting Information.
- 10Experimental details of the structure determination can be found in the Supporting Information. CCDC-661204 (3) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11Henkel and co-workers have also recently reported an example of O2 activation and methyl-group hydroxylation in binculear copper complexes with bis(guanidine) ligands:
- 11aS. Herres, A. J. Heuwing, U. Florke, J. Schneider, G. Henkel, Inorg. Chim. Acta 2005, 358, 1089–1095;
- 11bS. Herres-Pawlis, U. Florke, G. Henkel, Eur. J. Inorg. Chem. 2005, 3815–3824.
- 12V. Raab, J. Kipke, O. Burghaus, J. Sundermeyer, Inorg. Chem. 2001, 40, 6964–6971.
- 13L. Quant Hatcher, K. D. Karlin, J. Biol. Inorg. Chem. 2004, 9, 669–683.
- 14
- 14aJ. L. McLain, J. Lee, J. T. Groves, Biomimetic Oxid. Catal. Transition Met. Complexes 2000, 91–169;
- 14bL. Que, Jr., Acc. Chem. Res. 2007, 40, 493–500;
- 14cW. J. Song, M. S. Seo, S. DeBeer George, T. Ohta, R. Song, M. J. Kang, T. Tosha, T. Kitagawa, E. I. Solomon, W. Nam, J. Am. Chem. Soc. 2007, 129, 1268–1277;
- 14dK. Qin, C. D. Incarvito, A. L. Rheingold, K. H. Theopold, J. Am. Chem. Soc. 2002, 124, 14008–14009.
- 15E. V Gauchenova, Dissertation, Philipps-Universität Marburg, Germany, 2006; accessible online at http://archiv.ub.uni-marburg.de/diss/z2006/0155/pdf/devg.pdf.
- 16D. P. Goldberg, Acc. Chem. Res. 2007, 40, 626–634.
- 17
- 17aL. Li, A. A. N. Sarjeant, M. A. Vance, L. N. Zakharov, A. L. Rheingold, E. I. Solomon, K. D. Karlin, J. Am. Chem. Soc. 2005, 127, 15360–15361;
- 17bK. Itoh, H. Hayashi, H. Furutachi, T. Matsumoto, S. Nagatomo, T. Tosha, S. Terada, S. Fujinami, M. Suzuki, T. Kitagawa, J. Am. Chem. Soc. 2005, 127, 5212–5223.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.