What is Amphidinolide V? Report on a Likely Conquest†
Alois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorOleg Larionov Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorSusanne Flügge Dipl.-Chem.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorAlois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorOleg Larionov Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorSusanne Flügge Dipl.-Chem.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr, Germany, Fax: (+49) 208-306-2994
Search for more papers by this authorGenerous financial support from the MPG and the Fonds der Chemischen Industrie (Kekulé fellowship to S.F.) is gratefully acknowledged. We thank Prof. J.-I. Kobayashi for an exchange of information.
Graphical Abstract
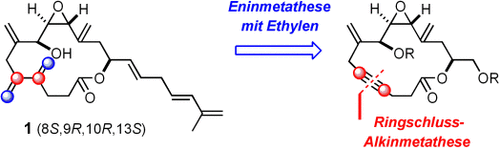
Die beeindruckende Leistungsfähigkeit der Metathese zeigt sich in der Synthese der für den cytotoxischen Naturstoff Amphidinolid V vorgeschlagenen Struktur sowie aller anderen Stereomere mit einer trans-Epoxideinheit. Aus dem erhaltenen umfangreichen Datensatz lässt sich folgern, dass die 8S,9R,10R,13S-konfigurierte Verbindung 1 höchstwahrscheinlich Amphidinolid V ist, auch wenn ein 1H-NMR-Signal vom beschriebenen Wert abweicht.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701640_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Kobayashi, M. Tsuda, Nat. Prod. Rep. 2004, 21, 77–93.
- 2For the synthesis of various amphidinolides, see Ref. [3] and the following for leading references:
- 2aD. R. Williams, W. S. Kissel, J. Am. Chem. Soc. 1998, 120, 11198–11199;
- 2bD. R. Williams, B. J. Myers, L. Mi, Org. Lett. 2000, 2, 945–948;
- 2cD. R. Williams, K. G. Meyer, J. Am. Chem. Soc. 2001, 123, 765–766;
- 2dH. W. Lam, G. Pattenden, Angew. Chem. 2002, 114, 526–529;
Angew. Chem. Int. Ed. 2002, 41, 508–511;
10.1002/1521-3773(20020201)41:3<508::AID-ANIE508>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 2eR. E. Maleczka, Jr., L. R. Terrell, F. Geng, J. S. Ward, Org. Lett. 2002, 4, 2841–2844;
- 2fB. M. Trost, P. E. Harrington, J. D. Chisholm, S. T. Wrobleski, J. Am. Chem. Soc. 2005, 127, 13598–13610;
- 2gB. M. Trost, S. T. Wrobelski, J. D. Chisholm, P. E. Harrington, M. Jung, J. Am. Chem. Soc. 2005, 127, 13589–13597;
- 2hP. Va, W. R. Roush, J. Am. Chem. Soc. 2006, 128, 15960–15961;
- 2iC. H. Kim, H. J. An, W. K. Shin, W. Yu, S. K. Woo, S. K. Jung, E. Lee, Angew. Chem. 2006, 118, 8187–8189; Angew. Chem. Int. Ed. 2006, 45, 8019–8021;
- 2jK. C. Nicolaou, W. E. Brenzovich, P. G. Bulger, T. M. Francis, Org. Biomol. Chem. 2006, 4, 2119–2157;
- 2kK. C. Nicolaou, P. G. Bulger, W. E. Brenzovich, Org. Biomol. Chem. 2006, 4, 2158–2183;
- 2lB. M. Trost, J. P. N. Papillon, T. Nussbaumer, J. Am. Chem. Soc. 2005, 127, 17921–17937;
- 2mA. K. Ghosh, G. Gong, J. Org. Chem. 2006, 71, 1085–1093;
- 2nE. A. Colby, K. C. O'Brien, T. F. Jamison, J. Am. Chem. Soc. 2005, 127, 4297–4307;
- 2oE. A. Colby, T. F. Jamison, Org. Biomol. Chem. 2005, 3, 2675–2684;
- 2pL.-S. Deng, X.-P. Huang, G. Zhao, J. Org. Chem. 2006, 71, 4625–4635.
- 3
- 3aA. Fürstner, E. Kattnig, O. Lepage, J. Am. Chem. Soc. 2006, 128, 9194–9204;
- 3bC. Aïssa, R. Riveiros, J. Ragot, A. Fürstner, J. Am. Chem. Soc. 2003, 125, 15512–15520;
- 3cO. Lepage, E. Kattnig, A. Fürstner, J. Am. Chem. Soc. 2004, 126, 15970–15971;
- 3dA. Fürstner, C. Aïssa, R. Riveiros, J. Ragot, Angew. Chem. 2002, 114, 4958–4960;
10.1002/ange.200290041 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4763–4766.
- 4T. Kubota, M. Tsuda, J. Kobayashi, Tetrahedron Lett. 2000, 41, 713–716.
- 5
- 5aA. Fürstner, C. Nevado, M. Tremblay, C. Chevrier, F. Teplý, C. Aïssa, M. Waser, Angew. Chem. 2006, 118, 5969–5974;
10.1002/ange.200601860 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5837–5842;
- 5bA. Fürstner, C. Aïssa, C. Chevrier, F. Teplý, C. Nevado, M. Tremblay, Angew. Chem. 2006, 118, 5964–5969;
10.1002/ange.200601859 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5832–5837;
- 5cA. Fürstner, M. M. Domostoj, B. Scheiper, J. Am. Chem. Soc. 2006, 128, 8087–8094;
- 5dA. Fürstner, L. Turet, Angew. Chem. 2005, 117, 3528–3532;
10.1002/ange.200500390 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3462–3466;
- 5eA. Fürstner, M. D. B. Fenster, B. Fasching, C. Godbout, K. Radkowski, Angew. Chem. 2006, 118, 5632–5636;
10.1002/ange.200601654 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5506–5510;
- 5fA. Fürstner, M. D. B. Fenster, B. Fasching, C. Godbout, K. Radkowski, Angew. Chem. 2006, 118, 5636–5641;
10.1002/ange.200601655 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5510–5515;
- 5gA. Fürstner, D. De Souza, L. Turet, M. D. B. Fenster, L. Parra-Rapado, C. Wirtz, R. Mynott, Chem. Eur. J. 2007, 13, 115–134;
- 5hA. Fürstner, D. Kirk, M. D. B. Fenster, C. Aïssa, D. De Souza, C. Nevado, C. T. T. Tuttle, W. Thiel, O. Müller, Chem. Eur. J. 2007, 13, 135–149, and references therein.
- 6
- 6aA. Fürstner, G. Seidel, Angew. Chem. 1998, 110, 1758–1760;
10.1002/(SICI)1521-3757(19980619)110:12<1758::AID-ANGE1758>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1734–1736;10.1002/(SICI)1521-3773(19980703)37:12<1734::AID-ANIE1734>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 6bA. Fürstner, O. Guth, A. Rumbo, G. Seidel, J. Am. Chem. Soc. 1999, 121, 11108–11113;
- 6cfor a review, see A. Fürstner, P. W. Davies, Chem. Commun. 2005, 2307–2320.
- 7
- 7aK. Tonogaki, M. Mori, Tetrahedron Lett. 2002, 43, 2235–2238;
- 7bM. Mori, Adv. Synth. Catal. 2007, 349, 121–135;
- 7cS. T. Diver, A. J. Giessert, Chem. Rev. 2004, 104, 1317–1382.
- 8For details, see the Supporting Information.
- 9
- 9aG. A. Molander, L. A. Felix, J. Org. Chem. 2005, 70, 3950–3956;
- 9bG. A. Molander, N. Ellis, Acc. Chem. Res. 2007, 40, 275–286.
- 10R. A. Johnson, K. B. Sharpless in Catalytic Asymmetric Synthesis, 2nd ed. ), Wiley-VCH, New York, 2000, pp. 231–280.
- 11T. Shibata, K. Nakatsui, K. Soai, Inorg. Chim. Acta 1999, 296, 33–36.
- 12
- 12aA. Fürstner, C. Mathes, C. W. Lehmann, J. Am. Chem. Soc. 1999, 121, 9453–9454;
- 12bA. Fürstner, C. Mathes, C. W. Lehmann, Chem. Eur. J. 2001, 7, 5299–5317;
10.1002/1521-3765(20011217)7:24<5299::AID-CHEM5299>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 12cA. Fürstner, K. Grela, C. Mathes, C. W. Lehmann, J. Am. Chem. Soc. 2000, 122, 11799–11805.
- 13P. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563–2585.
- 14Not considered were isomers with different double-bond geometries as well as isomers containing a cis-epoxide unit. The presence of the latter was ruled out on the basis of the 3JH9,H10 coupling constant of 2.2 Hz reported for natural amphidinolide V, which is characteristic of a trans-epoxide, see Ref. [4]. This assignment was corroborated by the NMR data of the synthetic trans epoxides prepared during this investigation, the corresponding coupling constants of which were invariably about 2 Hz.
- 15M. Taniguchi, H. Fujii, K. Oshima, K. Utimoto, Tetrahedron 1995, 51, 679–686.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.