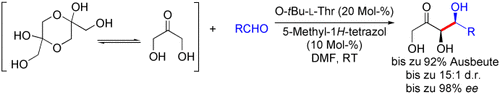Mimicking Fructose and Rhamnulose Aldolases: Organocatalytic syn-Aldol Reactions with Unprotected Dihydroxyacetone†
S. S. V. Ramasastry Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorKlaus Albertshofer
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorNaoto Utsumi Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorFujie Tanaka Prof. Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorCarlos F. Barbas III Prof. Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorS. S. V. Ramasastry Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorKlaus Albertshofer
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorNaoto Utsumi Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorFujie Tanaka Prof. Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorCarlos F. Barbas III Prof. Dr.
The Skaggs Institute for Chemical Biology and the Departments of Chemistry and Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA, Fax: (+1) 858-784-2583
Search for more papers by this authorThis study was supported in part by The Skaggs Institute for Chemical Biology.
Graphical Abstract
Nachahmende Amine: Aminosäuren mit primären Aminfunktionen katalysieren syn-selektive asymmetrische Aldolreaktionen zwischen nichtgeschütztem Dihydroxyaceton und mehreren aromatischen und aliphatischen Aldehyden (siehe Schema). Die vorgestellte Strategie ahmt die Wirkung der L-Rhamnulose-1-phosphat- und D-Fructose-1,6-Diphosphat-Aldolasen nach.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701269_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. M. Wrodnigg, F. K. Sprenger, Mini-Rev. Med. Chem. 2004, 4, 437–459;
- 1bD. B. Werz, P. H. Seeberger, Chem. Eur. J. 2005, 11, 3194–3206;
- 1cJ. J. Barchi, Jr., Curr. Pharm. Des. 2000, 6, 485–501.
- 2
- 2aK. C. Nicolaou, H. J. Mitchell, Angew. Chem. 2001, 113, 1624–1672;
10.1002/1521-3757(20010504)113:9<1624::AID-ANGE16240>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1576–1624;10.1002/1521-3773(20010504)40:9<1576::AID-ANIE15760>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 2bP. H. Seeberger, D. B. Werz, Nat. Rev. Drug Discovery 2005, 4, 751–763;
- 2cP. Sears, C.-H. Wong, Science 2001, 291, 2344–2350;
- 2dS. Hanessian in Preparative Carbohydrate Chemistry, Marcel Dekker, New York, 1997;
10.1201/9781482273588 Google Scholar
- 2eT. Hudlicky, D. A. Entwistle, K. K. Pitzer, A. J. Thorpe, Chem. Rev. 1996, 96, 1195–1220;
- 2fS. J. Danishefsky, K. F. McClure, J. T. Randolph, R. B. Ruggeri, Science 1993, 260, 1307–1309;
- 2gS. Y. Ko, A. W. M. Lee, S. Masamune, L. A. Reed III, K. B. Sharpless, F. J. Walker, Science 1983, 220, 949–951.
- 3
- 3aC.-H. Wong, M. C. Bryan, P. T. Nyffeler, H. Liu, E. Chapman, Pure Appl. Chem. 2003, 75, 179–186;
- 3bK. M. Koeller, C.-H. Wong, Nature 2001, 409, 232–240;
- 3cC.-H. Wong, T. D. Machajewski, Angew. Chem. 2000, 112, 1406–1430;
Angew. Chem. Int. Ed. 2000, 39, 1352–1375;
10.1002/(SICI)1521-3773(20000417)39:8<1352::AID-ANIE1352>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 3dW. D. Fessner, V. Helaine, Curr. Opin. Biotechnol. 2001, 12, 574–586;
- 3eD. Franke, T. Machajewski, C. C. Hsu, C.-H. Wong, J. Org. Chem. 2003, 68, 6828–6831.
- 4W. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580.
- 5For reviews, see:
- 5aM. Limbach, Chem. Biodiversity 2005, 2, 825–836;
- 5bU. Kazmaier, Angew. Chem. 2005, 117, 2224–2226;
10.1002/ange.200462873 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2186–2188;
- 5cF. Tanaka, C. F. Barbas III in Enantioselective Organocatalysis, Reactions and Experimental Procedures (Ed.: ), Wiley-VCH, Weinheim, 2007, pp. 19–55.
10.1002/9783527610945.ch2a Google Scholar
- 6For selected references, see:
- 6aN. S. Chowdari, D. B. Ramachary, A. Cordova, C. F. Barbas III, Tetrahedron Lett. 2002, 43, 9591;
- 6bA. B. Northrup, D. W. C. MacMillan, Science 2004, 305, 1752–1755;
- 6cD. Enders, C. Grondal, Angew. Chem. 2005, 117, 1235–1238;
10.1002/ange.200462428 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1210–1212;
- 6dJ. T. Suri, D. B. Ramachary, C. F. Barbas III, Org. Lett. 2005, 7, 1383–1385;
- 6eD. Enders, C. Grondal, M. Vrettou, G. Raabe ; Angew. Chem. 2005, 117 4147–4151;
10.1002/ange.200500810 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4079–4083;
- 6fJ. T. Suri, S. Mitsumori, K. Albertshofer, F. Tanaka, C. F. Barbas III, J. Org. Chem. 2006, 71, 3822–3828;
- 6gD. Enders, M. Vrettou, Synthesis 2006, 2155–2158;
- 6hC. Grondal, D. Enders, Tetrahedron 2006, 62, 329–337;
- 6iD. Enders, J. Palecek, C. Grondal, Chem. Commun. 2006, 655–657;
- 6jD. Enders, T. Gasperi, Chem. Commun. 2007, 88–90;
- 6kC. Grondal, D. Enders, Adv. Synth. Catal. 2007, 349, 694–702.
- 7For a suggested terminology, see: D. Enders, M. Voith, A. Lenzen, Angew. Chem. 2005, 117, 1330–1351;
10.1002/ange.200400659 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1304–1325.
- 8
- 8aS. S. V. Ramasastry, H. Zhang, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2007, 129, 288–289; for other syn-selective organocatalytic aldol reactions that followed, see:
- 8bT. Kano, Y. Yamaguchi, Y. Tanaka, K. Maruoka, Angew. Chem. 2007, 119, 1768–1770;
10.1002/ange.200604640 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1738–1740;
- 8cS. Luo, H. Xu, J. Li, L. Zhang, J.-P. Cheng, J. Am. Chem. Soc. 2007, 129, 3074–3075;
- 8dsurprisingly, 3S, 4R aldol products have been reported by using L-threonine-based catalysts, compared to the 3R, 4S products prepared here, and it has been stated that the free hydroxyacetone is not a reactive substrate, see: X. Wu, Z. Jiang, H.-M. Shen, Y. Lu, Adv. Synth. Catal. 2007, 349, 812–816.
- 9
- 9aA. Córdova, W. Notz, C. F. Barbas III, Chem. Commun. 2002, 3024–3025;
- 9bA. Córdova, W. Zou, P. Dziedzic, I. Ibrahem, E. Reyes, Y. Xu, Chem. Eur. J. 2006, 12, 5175 and A. Córdova, W. Zou, P. Dziedzic, I. Ibrahem, E. Reyes, Y. Xu, Chem. Eur. J. 2006, 12, 5383–5397.
- 10The price per gram in US dollars is $0.39, $93.00, and $4320.00 for the 1,3-dihydroxyacetone dimer, 2,2-dimethyl-1,3-dioxan-5-one, and dihydroxyacetone phosphate, respectively.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.