Highly Stable Pyrimidine-Motif Triplex Formation at Physiological pH Values by a Bridged Nucleic Acid Analogue†
S. M. Abdur Rahman Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
On leave from the Faculty of Pharmacy, Dhaka University, Dhaka-1000, Bangladesh
Search for more papers by this authorSayori Seki
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorSatoshi Obika Dr.
Graduate School of Pharmaceutical Sciences, Osaka University
PRESTO, JST, 4-1-8 Honcho Kawaguchi, Saitama 332-0012, Japan
Search for more papers by this authorSunao Haitani
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorKazuyuki Miyashita Prof.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorTakeshi Imanishi Prof.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorS. M. Abdur Rahman Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
On leave from the Faculty of Pharmacy, Dhaka University, Dhaka-1000, Bangladesh
Search for more papers by this authorSayori Seki
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorSatoshi Obika Dr.
Graduate School of Pharmaceutical Sciences, Osaka University
PRESTO, JST, 4-1-8 Honcho Kawaguchi, Saitama 332-0012, Japan
Search for more papers by this authorSunao Haitani
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorKazuyuki Miyashita Prof.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorTakeshi Imanishi Prof.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8204
Search for more papers by this authorS.M.A.R. thanks the Japan Society of Promotion of Science (JSPS) for financial support.
Graphical Abstract
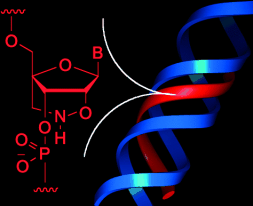
Aller guten Dinge sind drei: Eine neuartige überbrückte Nucleinsäure wurde entwickelt, in der die Furanose durch eine sechsgliedrige Brückeneinheit mit einer N-O-Bindung in einer Konformation vom N-Typ fixiert ist (siehe Bild). Oligonucleotide, die aus diesem Rest aufgebaut sind, bilden bei physiologischen pH-Werten stabile Triplexe.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z604857_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. E. Moser, P. B. Dervan, Science 1987, 238, 645;
- 1bM. D. Frank-Kamenetskii, S. M. Mirkin, Annu. Rev. Biochem. 1995, 64, 65;
- 1cV. N. Soyfer, V. N. Potaman, Triple-Helical Nucleic Acids, Springer, New York, 1996, p. 220;
10.1007/978-1-4612-3972-7_6 Google Scholar
- 1dM. M. Seidman, P. M. Glazer, J. Clin. Invest. 2003, 112, 487;
- 1eS. Buchini, C. J. Leumann, Curr. Opin. Chem. Biol. 2003, 7, 717.
- 2T. Imanishi, S. Obika, Chem. Commun. 2002, 1653.
- 3S. Obika, D. Nandu, Y. Hari, K. Morio, Y. In, T. Ishida, T. Imanishi, Tetrahedron Lett. 1997, 38, 8735.
- 4S. K. Singh, P. Nielsen, A. A. Koshkin, J. Wengel, Chem. Commun. 1998, 455.
- 5
- 5aS. Obika, Y. Hari, T. Sugimoto, M. Sekiguchi, T. Imanishi, Tetrahedron Lett. 2000, 41, 8923;
- 5bS. Obika, T. Uneda, T. Sugimoto, D. Nanbu, T. Minami, T. Doi, T. Imanishi, Bioorg. Med. Chem. 2001, 9, 1001;
- 5cH. Torigoe, Y. Hari, M. Sekiguchi, S. Obika, T. Imanishi, J. Biol. Chem. 2001, 276, 2354;
- 5dS. Obika, Y. Hari, M. Sekiguchi, T. Imanishi, Angew. Chem. 2001, 113, 2149;
10.1002/1521-3757(20010601)113:11<2149::AID-ANGE2149>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2079;10.1002/1521-3773(20010601)40:11<2079::AID-ANIE2079>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 5eS. Obika, Y. Hari, M. Sekiguchi, T. Imanishi, Chem. Eur. J. 2002, 8, 4796.
10.1002/1521-3765(20021018)8:20<4796::AID-CHEM4796>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 6
- 6aK. Morita, C. Hasegawa, M. Kaneko, S. Tsutsumi, J. Sone, T. Ishikawa, T. Imanishi, M. Koizumi, Bioorg. Med. Chem. Lett. 2002, 12, 73;
- 6bM. Koizumi, K. Morita, M. Daigo, S. Tsutsumi, K. Abe, S. Obika, T. Imanishi, Nucleic Acids Res. 2003, 31, 3267.
- 7This BNA is defined as 2′,4′-BNANC because the bridge between 2′-O and 4′-C is constituted by N and C atoms.
- 8The other fully modified TFOs formed triplexes at around the physiological pH value, see:
- 8aM. Bolli, C. Leumann, Angew. Chem. 1995, 107, 694;
10.1002/ange.19951070622 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 694;
- 8bC. Escudé, C. Giovannangeli, J.-S. Sun, D. H. Lloyd, J- K. Chen, S. M. Gryaznov, T. Garestier, C. Hélène, Proc. Natl. Acad. Sci. USA 1996, 93, 4365;
- 8cB. Cuenoud, F. Casset, D. Husken, F. Natt, R. M. Wolf, K.-H. Altmann, P. Martin, H. E. Moser, Angew. Chem. 1998, 110, 1350;
10.1002/(SICI)1521-3757(19980504)110:9<1350::AID-ANGE1350>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1288;10.1002/(SICI)1521-3773(19980518)37:9<1288::AID-ANIE1288>3.0.CO;2-U CAS Web of Science® Google Scholar
- 8dS. M. Gryaznov, H. Winter, Nucleic Acids Res. 1998, 26, 4160;
- 8eN. Kumar, K. E. Nielsen, S. Maiti, M. Petersen, J. Am. Chem. Soc. 2006, 128, 14.
- 9
- 9aM. Petersen, J. Wengel, Trends Biotechnol. 2003, 21, 74;
- 9bJ. S. Jespen, J. Wengel, Curr. Opin. Drug Discovery Dev. 2004, 7, 188;
- 9cB. Vester, J. Wengel, Biochemistry 2004, 43, 13233;
- 9dL. Wang, C. J. Yang, C. D. Medley, S. A. Benner, W. Tan, J. Am. Chem. Soc. 2005, 127, 15664.
- 10A. A. Koshkin, V. K. Rajwanshi, J. Wengel, Tetrahedron Lett. 1998, 39, 4381.
- 11We had to replace the benzyl groups with a cyclic disiloxy group because we experienced cleavage of the NO bridged structure during debenzylation of the corresponding dibenzyl derivative of 9.
- 12
- 12aK. Shah, H. Wu, T. M. Rana, Bioconjugate Chem. 1994, 5, 508;
- 12bM. Scherr, C. Klebba, R. Häner, A. Ganser, J. W. Engels, Bioorg. Med. Chem. Lett. 1997, 7, 1791.
- 13Similar triplex-to-single-strand dissociation was also reported recently by Leumann, see: S. Buchini, C. J. Leumann, Angew. Chem. 2004, 116, 4015;
10.1002/ange.200460159 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3925.
- 14Similar results were obtained by using two different BNA–TFOs in our previous investigation. The TFOs also failed to bind with a 30-bp target duplex.[5b]
- 15The Tm value of the corresponding ENA–TFO could not be determined because of the difficulties in purification of the TFO.
- 16In the case of room temperature EMSA, the triplexes were warmed to room temperature after incubating at 4 °C and kept at room temperature for two hours before applying onto the gel. The gel was run at room temperature at a constant voltage of 70 V.
- 17This type of lower mobility was also found in the case of the recently reported α-L-LNA-modified triplexes.[8e] Variation of triplex mobility was also noted by Roberts and Crothers, see: R. W. Roberts, D. M. Crothers, Science 1992, 258, 1463.
- 18J. L. Asensio, R. Carr, T. Brown, A. N. Lane, J. Am. Chem. Soc. 1999, 121, 11063.
- 19S. Rhee, Z.-J. Han, K. Liu, H. T. Miles, D. R. Davis, Biochemistry 1999, 38, 16810.
- 20T. P. Prakash, A. Puschl, E. Lesnik, V. Mohan, V. Tereshko, M. Egli, M. Manoharan, Org. Lett. 2004, 6, 1971.
- 21A. Mayer, A. Häberli, C. Leumann, Org. Biomol. Chem. 2005, 3, 1653.
- 22C. H. Gotfredsen, P. Schultze, J. Feigon, J. Am. Chem. Soc. 1998, 120, 4281.
- 23Rules for designing BNA–TFOs for optimum triplex-forming ability were described, see: B. W. Sun, B. R. Babu, M. D. Sørensen, K. Zakrzewska, J. Wengel, Biochemistry 2004, 43, 4160.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.