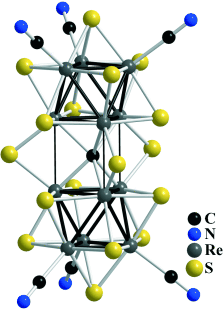
[Re12CS17(CN)6]n− (n=6, 8): A Sulfido–Cyanide Rhenium Cluster with an Interstitial Carbon Atom†
Yuri V. Mironov Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorNikolai G. Naumov Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorSvetlana G. Kozlova Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorSung-Jin Kim Prof.
Department of Chemistry, Ewha Womans University, Seoul 120-750, Korea, Fax: (+82) 2-3277-2384
Search for more papers by this authorVladimir E. Fedorov Prof.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorYuri V. Mironov Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorNikolai G. Naumov Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorSvetlana G. Kozlova Dr.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorSung-Jin Kim Prof.
Department of Chemistry, Ewha Womans University, Seoul 120-750, Korea, Fax: (+82) 2-3277-2384
Search for more papers by this authorVladimir E. Fedorov Prof.
Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3, Acad. Lavrentiev pr., 630090 Novosibirsk, Russia, Fax: (+7) 3833-309489
Search for more papers by this authorThis work was supported by the Russian Foundation for Basic Research (grants 05-03-08090 and 05-03-32123), the International Cooperation Research Program of the Ministry of Science & Technology of Korea (grant M60403000099-04A0100-02310), and the Division of Chemistry and Sciences on Materials of RAS (project No. 15 of Program 4.1). The authors are grateful to the Boreskov Institute of Catalysis SB RAS for an opportunity to use the ADF program.
Graphical Abstract
References
- 1
- 1aY. V. Mironov, A. V. Virovets, V. E. Fedorov, N. V. Podberezskaya, O. V. Shishkin, Y. T. Struchkov, Polyhedron 1995, 14, 3171–3173;
- 1bA. Slougui, Y. V. Mironov, A. Perrin, V. E. Fedorov, Croat. Chem. Acta 1995, 68, 885–890.
- 2N. G. Naumov, A. V. Virovets, N. V. Podberezskaya, V. E. Fedorov, J. Struct. Chem. (Engl. Transl.) 1997, 38, 857–862.
- 3Y. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt, J. A. Ibers, J. Am. Chem. Soc. 1997, 119, 493–498.
- 4N. G. Naumov, E. V. Ostanina, A. V. Virovets, M. Schmidtman, A. Müller, V. E. Fedorov, Russ. Chem. Bull. 2002, 51, 866–871.
- 5aT. Yoshimura, S. Ishizaka, Y. Sasaki, H. B. Kim, N. Kitamura, N. G. Naumov, M. N. Sokolov, V. E. Fedorov, Chem. Lett. 1999, 1121–1122;
- 5bS. A. Baudron, A. Deluzet, K. Boubekeur, P. Batail, Chem. Commun. 2002, 2124–2125.
- 6L. G. Beauvais, M. P. Shores, J. R. Long, Chem. Mater. 1998, 10, 3783–3786.
- 7
- 7aN. G. Naumov, A. V. Virovets, M. N. Sokolov, S. B. Artemkina, V. E. Fedorov, Angew. Chem. 1998, 110, 2043–2045;
10.1002/(SICI)1521-3757(19980703)110:13/14<2043::AID-ANGE2043>3.0.CO;2-G Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1943–1945;10.1002/(SICI)1521-3773(19980803)37:13/14<1943::AID-ANIE1943>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 7bN. G. Naumov, A. V. Virovets, V. E. Fedorov, J. Struct. Chem. (Engl. Transl.) 2000, 41, 499–520;
- 7cN. G. Naumov, D. V. Soldatov, J. A. Ripmeester, S. B. Artemkina, V. E. Fedorov, Chem. Commun. 2001, 571–572;
- 7dY. V. Mironov, N. G. Naumov, K. A. Brylev, O. A. Efremova, V. E. Fedorov, K. Hegetschweiler, Angew. Chem. 2004, 116, 1317–1321;
10.1002/ange.200351595 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1297–1300;
- 7eL. G. Beauvais, M. P. Shores, J. R. Long, J. Am. Chem. Soc. 2000, 122, 2763–2772;
- 7fM. V. Bennett, L. G. Beauvais, M. P. Shores, J. R. Long, J. Am. Chem. Soc. 2001, 123, 8022–8032;
- 7gY. Kim, S. M. Park, W. Nam, S. J. Kim, Chem. Commun. 2001, 1470–1471;
- 7hY. Kim, S. Kim, S. J. Kim, M. K. Lee, M. Kim, H. Lee, C. S. Chin, Chem. Commun. 2004, 1692–1693.
- 8H. Imoto, N. G. Naumov, A. V. Virovets, T. Saito, V. E. Fedorov, J. Struct. Chem. (Engl. Transl.) 1998, 39, 720–727.
- 9Y. V. Mironov, V. E. Fedorov, C. C. McLauchlan, J. A. Ibers, Inorg. Chem. 2000, 39, 1809–1811.
- 10The reaction of ReS2 with KCN has been studied in the temperature range from 650 to 800 °C by using different ReS2/KCN ratios. We found that for mass ratios of ReS2/KCN from 1:2 to 1:20 the main product of the reaction was K8[Re12CS17(CN)6]. To understand the conditions that yield [Re12CS17(CN)6]8−, KCs3[Re6S8(CN)6] was used as the starting material. It was found that [Re6S8(CN)6]4− transforms into [Re12CS17(CN)6]8− if the temperature rises to between 700 and 800 °C. Furthermore, the reaction of [Re6S8Br2] with KCN at high temperature also gave the [Re12CS17(CN)6]8− cluster , whereas below 650 °C [Re6S8(CN)6]4− formed. These data indicate that [Re12CS17(CN)6]8− cluster is thermodynamically stable within the 650 to 800 °C range.
- 11X-ray structural analyses: Bruker SMART CCD diffractometer with area detector, graphite monochromator, MoKα radiation (λ=0.71073 Å), SHELX-97 program[27] for structure solution (direct methods) and refinement (full-matrix least-squares on F 2). 1: C7K8N6Re12S17 (Mr=3260.35), crystal size 0.14×0.13×0.03 mm, monoclinic, space group P21/m, a=9.1806(11), b=29.210(2), c=9.2006(8) Å, β=119.777(1)o, V=2141.5(4) Å3, Z=2, ρcalcd=5.056 g cm−3, μ=35.381 mm−1, 2.55<θ< 28.21°, T=293(2) K, face-indexed absorption correction (transmission coefficient: 0.0831, 0.4598). Reflections: 13 011 collected, 4890 unique (Rint=0.0512), 3756 observed (I>2σ(I)). There were 239 parameters refined with R=0.0619 (I>2σ(I)), wR2=0.1693 (all data), GOF=1.107, residual electron density: +4.889/−4.827 e Å−3. 2: C7H40K6N6O20Re12S17 (Mr=3542.47), crystal size 0.12×0.12×0.03 mm, hexagonal, space group P63/mmc, a=10.8843(8), c=27.840(4) Å, V=2856.3(5) Å3, Z=2, ρcalcd=4.119 g cm−3, μ=26.427 mm−1, 2.16<θ<28.24°, T=293(2) K, face-indexed absorption correction (transmission coefficient: 0.1436, 0.5044). Reflections: 16 972 collected, 1383 unique (Rint=0.0384), 1236 observed (I>2σ(I)), 82 parameters refined with R=0.0263 (I>2σ(I)), wR2=0.0589 (all data), GOF=1.107, residual electron density: +1.643/−2.408 e Å−3. 3: C7Cs6N6Re12S17 (Mr=3746.34), crystal size 0.09×0.09×0.01 mm, hexagonal, space group P
 2m, a=9.7270(15), c=14.629(3) Å, V=1198.7(4) Å3, Z=1, ρcalcd=5.190 g cm−3, μ=35.407 mm−1, 2.42<θ<28.18°, T=293(2) K, face-indexed absorption correction (transmission coefficient: 0.1428, 0.6760). Reflections: 7284 collected, 1105 unique (Rint=0.0672), 913 observed (I>2σ(I)), 59 parameters refined with R=0.0340 (I>2σ(I)), wR2=0.0915 (all data), GOF=1.046, residual electron density: +2.391/−1.234 e Å−3. Further details on the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49) 7247-808-666; e-mail: [email protected]), on quoting the depository numbers CSD-415476–415478.
2m, a=9.7270(15), c=14.629(3) Å, V=1198.7(4) Å3, Z=1, ρcalcd=5.190 g cm−3, μ=35.407 mm−1, 2.42<θ<28.18°, T=293(2) K, face-indexed absorption correction (transmission coefficient: 0.1428, 0.6760). Reflections: 7284 collected, 1105 unique (Rint=0.0672), 913 observed (I>2σ(I)), 59 parameters refined with R=0.0340 (I>2σ(I)), wR2=0.0915 (all data), GOF=1.046, residual electron density: +2.391/−1.234 e Å−3. Further details on the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49) 7247-808-666; e-mail: [email protected]), on quoting the depository numbers CSD-415476–415478.
- 12S. S. Yarovoi, S. F. Solodovnikov, Y. V. Mironov, V. E. Fedorov, J. Struct. Chem. (Engl. Transl.) 2003, 44, 318–321.
- 13W. Bronger, H. J. Miessen, P. Müller, R. Neugröschel, J. Less-Common Met. 1985, 105, 303–310.
- 14W. Bronger, Metal Clusters in Chemistry, Vol. 3, (Eds.: ), Wiley-VCH, Weinheim, 1999, pp. 1591–1611.
10.1002/9783527618316.ch5f Google Scholar
- 15To determine isotropic chemical shifts of carbons the 13C magic-angle spinning (MAS) NMR measurements were made by using a BRUKER Bio-Spin Avance 400 MHz solid-state NMR spectrometer. The spinning rate was 20 kHz, the π/2 pulse duration was 5 s, the relaxation delay was 10 s. About 200 free induction decays were accumulated at room temperature. TMS was used as a reference. The 15N NMR spectrum contained one sharp signal at 311 ppm (relative to NH3), which corresponds to coordinated cyano ligands. No signal attributable to an interstitial nitrogen atom was found. The diamagnetic properties of compounds 1–3 indicated the presence of an interstitial atom with an even number of electrons.
- 16
- 16aAmsterdam Density Functional (ADF) program, Release 2003.02, Vrije Universteit, Amsterdam, The Netherlands, 2002;
- 16bG. Te-Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. Van-Gisbergen, J. G. Snijders, T. Ziegler, J. Comput. Chem. 2001, 22, 931.
- 17It is interesting to note that in rhenium cluster chemistry there are several types of cluster in which the rhenium atoms have a +III oxidation state; besides [Re12CS17(CN)6]8−, there are three well-known cluster complexes, namely, dinuclear [Re2X8]2− with quadruple ReRe bond, trigonal [Re3X12]3− with double ReRe bonds, and octahedral [Re6Q8X6]4− with single ReRe bonds (X=halide ions).
- 18F. Cecconi, C. A. Ghilardi, S. Midollini, A. Orlandini, Inorg. Chim. Acta 1993, 214, 13–15.
- 19S. Kamiguchi, H. Imoto, T. Saito, Chem. Lett. 1996, 555–556.
- 20S. Amari, H. Imoto, T. Saito, Chem. Lett. 1997, 967–968.
- 21Z. P. Zheng, R. H. Holm, Inorg. Chem. 1997, 36, 5173–5178.
- 22Z. P. Zheng, J. R. Long, R. H. Holm, J. Am. Chem. Soc. 1997, 119, 2163–2171.
- 23
- 23aY. Q. Zheng, H. G. von Schnering, J. H. Chang, Y. Grin, G. Engelhardt, G. Heckmann, Z. Anorg. Allg. Chem. 2003, 629, 1256–1264;
- 23bE. J. Welch, N. R. M. Crawford, R. G. Bergman, J. R. Long, J. Am. Chem. Soc. 2003, 125, 11464–11465;
- 23cE. J. Welch, C. L. Yu, N. R. M. Crawford, J. R. Long, Angew. Chem. 2005, 117, 2605–2609;
10.1002/ange.200462585 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2549–2553.
- 24H. Womelsdorf, H. J. Meyer, Angew. Chem. 1994, 106, 2022–2023; Angew. Chem. Int. Ed. Engl. 1994, 33, 1943–1944.
- 25H. J. Meyer, J. D. Corbett, Inorg. Chem. 1991, 30, 963–967.
- 26C. Perrin, M. Sergent, J. Chem. Res. 1983, 38–39.
- 27aG. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997;
- 27bG. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.