An Oriented 1D Coordination/Organometallic Dimetallic Molecular Wire with AgPd Metal–Metal Bonds†
Pierre Braunstein Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorCéline Frison
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorNicola Oberbeckmann-Winter Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorXavier Morise Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorAbdelatif Messaoudi
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorMarc Bénard Dr.
Laboratoire de Chimie Quantique, UMR 7551 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorMarie-Madeleine Rohmer Dr.
Laboratoire de Chimie Quantique, UMR 7551 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorRichard Welter Prof.
Laboratoire DECMET, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cedex, France
Crystal structure analysis.
Search for more papers by this authorPierre Braunstein Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorCéline Frison
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorNicola Oberbeckmann-Winter Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorXavier Morise Dr.
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorAbdelatif Messaoudi
Laboratoire de Chimie de Coordination, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorMarc Bénard Dr.
Laboratoire de Chimie Quantique, UMR 7551 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorMarie-Madeleine Rohmer Dr.
Laboratoire de Chimie Quantique, UMR 7551 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cédex, France
Search for more papers by this authorRichard Welter Prof.
Laboratoire DECMET, UMR 7513 CNRS, Institut Le Bel, Université Louis Pasteur, 4, rue Blaise Pascal, 67070 Strasbourg Cedex, France
Crystal structure analysis.
Search for more papers by this authorThis work was supported by the CNRS and the Ministère de la Recherche and the European Commission. N.O.-W. is grateful for a Marie Curie fellowship (HPMF-CT-2002-01659) and also to the French Embassy in Berlin and the Ministère des Affaires Etrangères (Paris) for a grant. M.B. and M.-M.R. thank the IDRIS computer center (Orsay, France) and the “GdR DFT” action from the CNRS.
Graphical Abstract
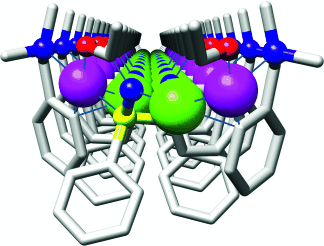
Ein subtiles elektronisches Gleichgewicht zwischen kovalenten und dativen Wechselwirkungen verbrückender Phosphanyliminolat-Liganden führt zur Bildung eines eindimensionalen Heterodimetall-Koordinationspolymers, in dem Pd-Ag-Wechselwirkungen vorliegen (siehe Bild; Pd lila, Ag grün). Die Anordnung der lipophilen aromatischen Ringe der unendlichen Zickzackketten bezüglich der Metallatome hat eine ungewöhnliche Schichtstruktur zur Folge.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461291_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For NiII complexes used in the catalytic oligomerization of ethylene, see, for example, P. Braunstein, Y. Chauvin, S. Mercier, L. Saussine, A. De Cian, J. Fischer, J. Chem. Soc. Chem. Commun. 1994, 2203.
- 2For PdII complexes used in the reversible coordination of CO2 and telomerization with butadiene, see P. Braunstein, D. Matt, D. Nobel, J. Am. Chem. Soc. 1988, 110, 3207.
- 3For RhI complexes in alkane activation, see P. Braunstein, Y. Chauvin, J. Nähring, A. DeCian, J. Fischer, A. Tiripicchio, F. Ugozzoli, Organometallics 1996, 15, 5551.
- 4
- 4aF. Balegroune, P. Braunstein, D. Grandjean, D. Matt, D. Nobel, Inorg. Chem. 1988, 27, 3320;
- 4bJ. Andrieu, P. Braunstein, A. Tiripicchio, F. Ugozzoli, Inorg. Chem. 1996, 35, 5975.
- 5
- 5aP. Braunstein, T. M. Gomes Carneiro, D. Matt, F. Balegroune, D. Grandjean, Organometallics 1989, 8, 1737;
- 5bF. Balegroune, P. Braunstein, T. M. Gomes Carneiro, D. Grandjean, D. Matt, J. Chem. Soc. Chem. Commun. 1989, 582.
- 6
- 6aP. Braunstein, D. Matt, D. Nobel, Chem. Rev. 1988, 88, 747;
- 6bP. Braunstein, D. Nobel, Chem. Rev. 1989, 89, 1927.
- 7J. Andrieu, P. Braunstein, M. Drillon, Y. Dusausoy, F. Ingold, P. Rabu, A. Tiripicchio, F. Ugozzoli, Inorg. Chem. 1996, 35, 5986.
- 8A. Apfelbacher, P. Braunstein, L. Brissieux, R. Welter, Dalton Trans. 2003, 1669.
- 9P. Braunstein, C. Frison, X. Morise, R. D. Adams, J. Chem. Soc. Dalton Trans. 2000, 2205.
- 10A. Affeld, G. M. Hübner, C. Steel, C. A. Schalley, Eur. J. Org. Chem. 2001, 2877.
- 11For full experimental data on the synthesis, spectroscopy, and elemental analysis of the complexes see the Supporting Information. X-ray structure analysis of 4⋅SO3CF3⋅0.5 CH2Cl2, 5⋅SO3CF3⋅2 CH2Cl2, and 6⋅SO3CF3: Selected crystals were mounted on a Nonius Kappa-CCD area detector diffractometer (MoKα, λ=0.71073 Å). The complete conditions of data collection (Denzo software) and structure refinements are given below. The cell parameters were determined from reflections taken from one set of ten frames (1.0° steps in phi angle), each at 20 s exposure. The structures were solved by direct methods (SHELXS97) and refined against F2 using the SHELXL97 software. The absorption was corrected empirically (with Sortav). All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were generated according to the stereochemistry and refined using a riding model in SHELXL97. 4⋅SO3CF3⋅0.5 CH2Cl2: C42H40AuF3N2O4P2PdS⋅0.5 CH2Cl2; Mr=1133.59, monoclinic, space group P21/c, a=9.379(3), b=41.299(5), c=12.061(3) Å, β=99.46(5)°, V=4608(2) Å3, Z=4, ρcalcd=1.634 g cm−3, μ=3.796 mm−1, F(000)=2228, T=173 K. Colorless crystal, dimensions 0.04×0.05×0.06 mm3. A total of 10 051 reflections were collected on a Kappa CCD diffractometer (phi scans), 1.78°<θ<27.50°, 10 051 independent reflections with 6631 having I>2σ(I); 532 parameters; R1=0.0482; wR2=0.1586, GOF=0.982, maximum residual electronic density=1.442 e Å−3. 5⋅SO3CF3⋅2 CH2Cl2: C24H25AgF3N2O4PPdS⋅2 CH2Cl2; Mr=909.61, monoclinic, space group P21/a, a=12.437(5), b=20.109(6), c=14.107(4) Å, β=106.52(5)°, V=3383(2) Å3, Z=4, ρcalcd=1.786 g cm−3, μ=1.586 mm−1, F(000)=1800. Colorless crystal, dimensions 0.08×0.10×0.13 mm3. A total of 14 300 reflections were collected on a Kappa CCD diffractometer (phi scans), 1.51°<θ<27.48°, 7719 independent reflections with 5826 having I>2σ(I); 395 parameters; R1=0.0397; wR2=0.1388, GOF=1.135, maximum residual electronic density=1.253 e Å−3. The figure shown in the Supporting Information was produced using the UCSF Chimera package from the Computer Graphics Laboratory, University of California, San Francisco: “Chimera: An Extensible Molecular Modeling Application Constructed Using Standard Components”: C. C. Huang, G. S. Couch, E. F. Pettersen, T. E. Ferrin, Pacific Symposium on Biocomputing 1996, 1, 724. 6⋅SO3CF3: C47H50AgF3N4O5P2Pd2S; Mr=1222.58, triclinic, space group P
 , a=13.169(1), b=14.722(1), c=14.769(1) Å, α=61.42(5)°, β=81.93(5)°, β=87.04(5)°, V=2489.2(3) Å3, Z=2, ρcalcd=1.631 g cm−3, μ=1.269 mm−1, F(000)=1224. Colorless crystal, dimensions 0.08×0.10×0.12 mm3. A total of 14 969 reflections were collected on a Kappa CCD diffractometer (phi scans), 1.56°<θ<30.33°, 14 471 independent reflections with 8498 having I>2σ(I); 586 parameters; R1=0.0545; wR2=0.1751, GOF=1.023, maximum residual electronic density=1.420 e− Å−3. CCDC-240188–240190 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
, a=13.169(1), b=14.722(1), c=14.769(1) Å, α=61.42(5)°, β=81.93(5)°, β=87.04(5)°, V=2489.2(3) Å3, Z=2, ρcalcd=1.631 g cm−3, μ=1.269 mm−1, F(000)=1224. Colorless crystal, dimensions 0.08×0.10×0.12 mm3. A total of 14 969 reflections were collected on a Kappa CCD diffractometer (phi scans), 1.56°<θ<30.33°, 14 471 independent reflections with 8498 having I>2σ(I); 586 parameters; R1=0.0545; wR2=0.1751, GOF=1.023, maximum residual electronic density=1.420 e− Å−3. CCDC-240188–240190 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 12
- 12aR. Uson, A. Laguna, M. Laguna, B. R. Manzano, P. G. Jones, G. M. Sheldrick, J. Chem. Soc. Dalton Trans. 1985, 2417;
- 12bP. A. Bella, O. Crespo, E. J. Fernandez, A. K. Fischer, P. G. Jones, A. Laguna, J. M. Lopez-de-Luzuriaga, M. Monge, J. Chem. Soc. Dalton Trans. 1999, 4009.
- 13The closest interactions between the triflate anion and the metal complex in 4⋅SO3CF3⋅0.5 CH2Cl2 involve atoms F3 and O2 of the former and H27 and H28 of an aryl group bound to P2 at 2.84(1) and 2.59(1) Å, respectively.
- 14P. Braunstein, C. Frison, X. Morise, C. R. Chim. 2002, 5, 131.
- 15In the crystal structure of (5⋅SO3CF3⋅2 CH2Cl2)n the triflate anion is positioned in the vicinity of the Ag+ ion but does not act as a ligand. The shortest intermolecular contacts are found between the triflate oxygen atom O3 and H7A and H13 (2.42(1) and 2.59(1) Å, respectively), and the fluorine atoms F1 and F3 and the methyl protons H11C and H11A (2.50(1) and 2.77(1) Å, respectively).
- 16P. Braunstein, T. M. Gomes Carneiro, D. Matt, A. Tiripicchio, M. Tiripicchio Camellini, Angew. Chem. 1986, 98, 721; Angew. Chem. Int. Ed. Engl. 1986, 25, 748.
- 17
- 17aJ. E. Kickham, S. J. Loeb, Organometallics 1995, 14, 3584;
- 17bW. Xu, R. J. Puddephatt, K. W. Muir, A. A. Torabi, Organometallics 1994, 13, 3054;
- 17cT. Yamaguchi, F. Yamazaki, T. Ito, J. Am. Chem. Soc. 2001, 123, 743.
- 18
- 18aFor reviews, see I. D. Salter, in Comprehensive Organometallic Chemistry II, Vol. 10 (Eds.: ), Elsevier, Oxford, 1995, p. 255;
10.1016/B978-008046519-7.00089-7 Google ScholarS.-M. Lee, W.-T. Wong, J. Cluster Sci. 1998, 9, 417;
- 18bY. Liu, K. H. Lee, J. J. Vittal, T. S. A. Hor, J. Chem. Soc. Dalton Trans. 2002, 2747;
- 18cE. Alonso, J. Forniés, C. Fortuño, A. Martin, A. G. Orpen, Organometallics 2003, 22, 5011.
- 19M. A. Carvajal, J. J. Novoa, S. Alvarez, J. Am. Chem. Soc. 2004, 126, 1465.
- 20J. C. Bailar in Preparative Inorganic Reactions, Vol. 1 (Ed.: ), Interscience, New York, 1964, pp. 1.
- 21For recent reviews, see C. Janiak, Dalton Trans. 2003, 2781; S. Kitagawa, R. Kitaura, S.-i. Noro, Angew. Chem. 2004, 116, 2388; Angew. Chem. Int. Ed. 2004, 43, 2334; and for AgI-based coordination polymers: A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk, M. Schröder, Coord. Chem. Rev. 2001, 222, 155.
- 22
- 22aJ. F. Berry, F. A. Cotton, L. M. Daniels, C. A. Murillo, X. Wang, Inorg. Chem. 2003, 42, 2418;
- 22bJ. F. Berry, F. A. Cotton, P. Lei, T. Lu, C. A. Murillo, Inorg. Chem. 2003, 42, 3534;
- 22cJ. F. Berry, F. A. Cotton, C. A. Murillo, Organometallics 2004, 23, 2503, and references therein.
- 23R. Clérac, F. A. Cotton, L. M. Daniels, K. R. Dunbar, K. Kirschbaum, C. A. Murillo, A. A. Pinkerton, A. J. Schultz, X. Wang, J. Am. Chem. Soc. 2000, 122, 6226.
- 24J. K. Bera, K. R. Dunbar, Angew. Chem. 2002, 114, 4633;
10.1002/1521-3757(20021202)114:23<4633::AID-ANGE4633>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4453, and references therein.10.1002/1521-3773(20021202)41:23<4453::AID-ANIE4453>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 25
- 25aI. P. Y. Shek, W.-Y. Wong, T.-C. Lau, New J. Chem. 2000, 24, 733;
- 25bS.-M. Peng, C.-C. Wang, Y.-L. Jang, Y.-H. Chen, F.-Y. Li, C.-Y. Mou, M.-K. Leung, J. Magn. Magn. Mater. 2000, 209, 80;
- 25cT. Nakajima, A. Ishiguro, Y. Wakatsuki, Angew. Chem. 2001, 113, 1096;
10.1002/1521-3757(20010316)113:6<1096::AID-ANGE10960>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1066;10.1002/1521-3773(20010316)40:6<1066::AID-ANIE10660>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 25dF. Jalilehvand, L. Eriksson, J. Glaser, M. Maliarik, J. Mink, M. Sandström, I. Toth, J. Toth, Chem. Eur. J. 2001, 7, 2167;
10.1002/1521-3765(20010518)7:10<2167::AID-CHEM2167>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 25eA. F. Heyduk, D. J. Krodel, E. E. Meyer, D. G. Nocera, Inorg. Chem. 2002, 41, 634;
- 25fC.-Y. Yeh, C.-H. Chou, K.-C. Pan, C.-C. Wang, G.-H. Lee, Y. O. Su, S.-M. Peng, J. Chem. Soc. Dalton Trans. 2002, 2670;
- 25gC. Tejel, M. A. Ciriano, B. E. Villarroya, J. A. Lopez, F. J. Lahoz, L. A. Oro, Angew. Chem. 2003, 115, 547;
10.1002/ange.200390120 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 530;
- 25hT. Sheng, R. Appelt, V. Comte, H. Vahrenkamp, Eur. J. Inorg. Chem. 2003, 3731;
- 25iE. J. Fernandez, J. M. Lopez-de-Luzuriaga, M. Monge, M. Montiel, M. E. Olmos, J. Pérez, A. Laguna, F. Mendizabal, A. A. Mohamed, J. P. Fackler, Jr., Inorg. Chem. 2004, 43, 3573.
- 26aM.-M. Rohmer, M. Bénard, Chem. Soc. Rev. 2001, 30, 340;
- 26bM.-M. Rohmer, A. Strich, M. Bénard, J.-P. Malrieu, J. Am. Chem. Soc. 2001, 123, 9126;
- 26cN. Benbellat, M.-M. Rohmer, M. Bénard, Chem. Commun. 2001, 2368;
- 26dF.-Y. Li, L. Chen, C.-Y. Mou, S.-M. Peng, Y. Wang, Int. J. Mol. Sci. 2002, 3, 38;
- 26eP. Kiehl, M.-M. Rohmer, M. Bénard, Inorg. Chem. 2004, 43, 3151.
- 27
- 27aF. A. Cotton, C. A. Murillo, D. J. Timmons, Chem. Commun. 1999, 1427;
- 27bF. A. Cotton, E. V. Dikarev, M. A. Petrukhina, J. Chem. Soc. Dalton Trans. 2000, 4241;
- 27cK.-T. Wong, J.-M. Lehn, S.-M. Peng, G.-H. Lee, Chem. Commun. 2000, 2259;
- 27dF. P. Pruchnik, P. Jakimowicz, Z. Ciunik, K. Stanislawek, L. A. Oro, C. Tejel, M. A. Ciriano, Inorg. Chem. Commun. 2001, 4, 19;
- 27eF. A. Cotton, C. Lin, C. A. Murillo, Chem. Commun. 2001, 11.
- 28J.-P. Lang, S.-J. Ji, Q.-F. Xu, Q. Shen, K. Tatsumi, Coord. Chem. Rev. 2003, 241, 47.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.